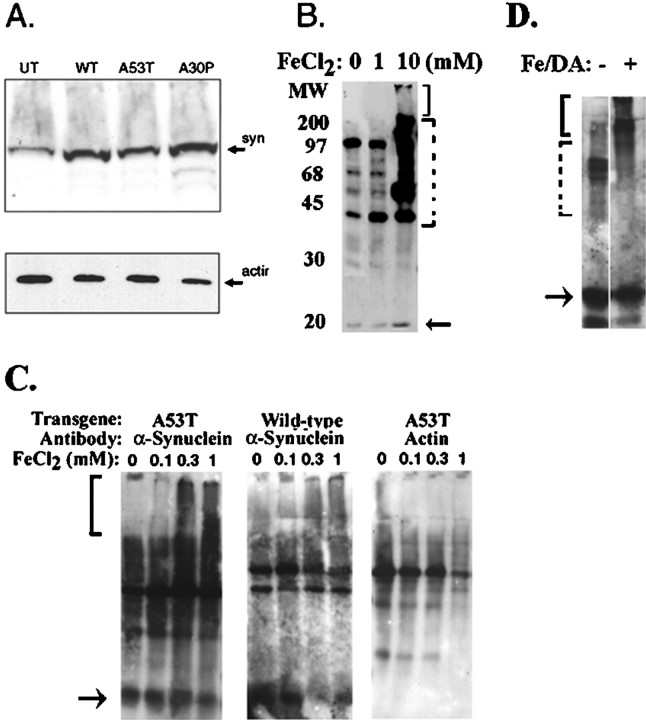
Fig. 1.
Iron stimulates α-synuclein aggregation.A, Immunoblot of α-synuclein in cell lines overexpressing wild-type (WT), A53T, and A30P α-synuclein using the monoclonal anti-α-synuclein antibody (top). The same immunoblot was then stripped and reprobed with antibody to actin. UT, Untransfected.B, Aggregation of α-synuclein in BE-M17 cells expressing A53T α-synuclein after treatment with 1 or 10 mm FeCl2 for 48 hr (in each panel, thesolid bracket shows putative aggregates in the stacking gel, and the dotted bracket shows putative aggregates in the separating gel). C, Longer exposure (4 d) enabled lower doses of FeCl2 to induce aggregation of α-synuclein BE-M17 cells expressing A53T α-synuclein (left) or wild-type α-synuclein (middle). Under these conditions, little or no aggregation of actin was observed (right). D, In vitroanalysis of α-synuclein aggregation. Immunoblotting of α-synuclein in brain membrane fractions under basal conditions (lane 1) or after treatment with 10 mm FeCl2and 500 μm dopamine (DA) for 24 hr (lane 2) shows the induction of high molecular weight aggregates similar to that seen in the BE-M17 cells.