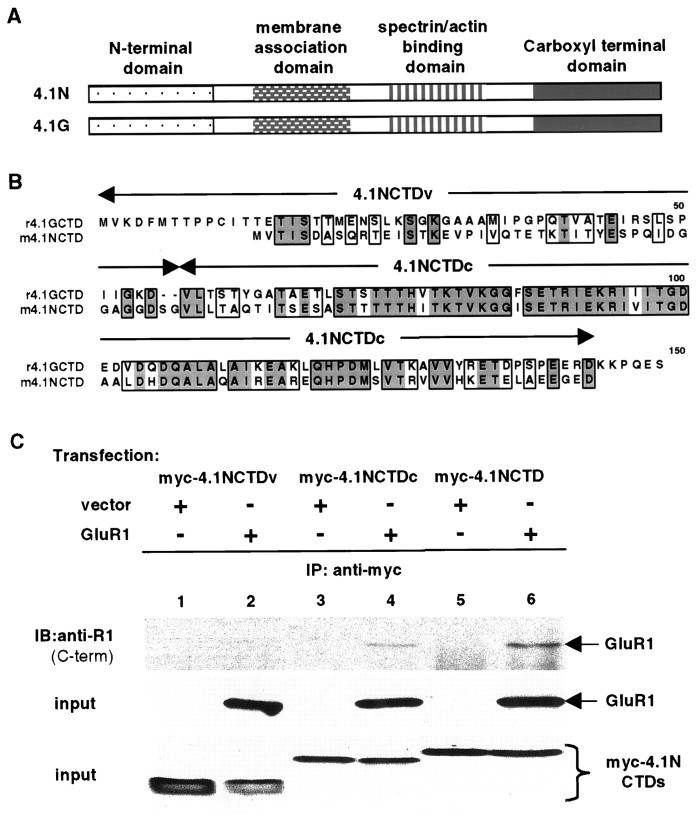
Fig. 4.
A consensus region within 4.1N is sufficient for interaction with GluR1. A, Schematic diagram of domain structures of 4.1N and 4.1G. The two proteins have significant homology at the membrane association domain, spectrin–actin binding domain, and CTD but have little homology at the N-terminal domain.B, Sequence alignment of CTDs of 4.1N and 4.1G. Identical amino acid residues are boxed anddarkly shaded; conserved residues areboxed and lightly shaded. The alignment shows that CTDs of the two proteins contain a variable N-terminal region (4.1NCTDv) and a highly conserved C-terminal region (4.1NCTDc). C, Association of the consensus region of 4.1NCTD with GluR1. A myc-tagged variable region (myc-4.1NCTDv; lanes 1, 2) or consensus region (myc-4.1NCTDc; lanes 3, 4) of 4.1NCTD or the entire 4.1NCTD (myc-4.1NCTD;lanes 5, 6) was co-transfected with vector (lanes 1, 3, 5) or with GluR1 (lanes 2, 4, 6) into 293T cells. Immunoprecipitations were performed on solubilized cell lysates using anti-myc antibody followed by SDS-PAGE and immunoblotting with anti-GluR1 antibody (top panel). The presence of GluR1 (middle panel) and myc-tagged proteins (bottom panel) in the input for immunoprecipitation was confirmed by Western blot using anti-GluR1 C-terminal and anti-myc antibodies, respectively.