Fig. 7.
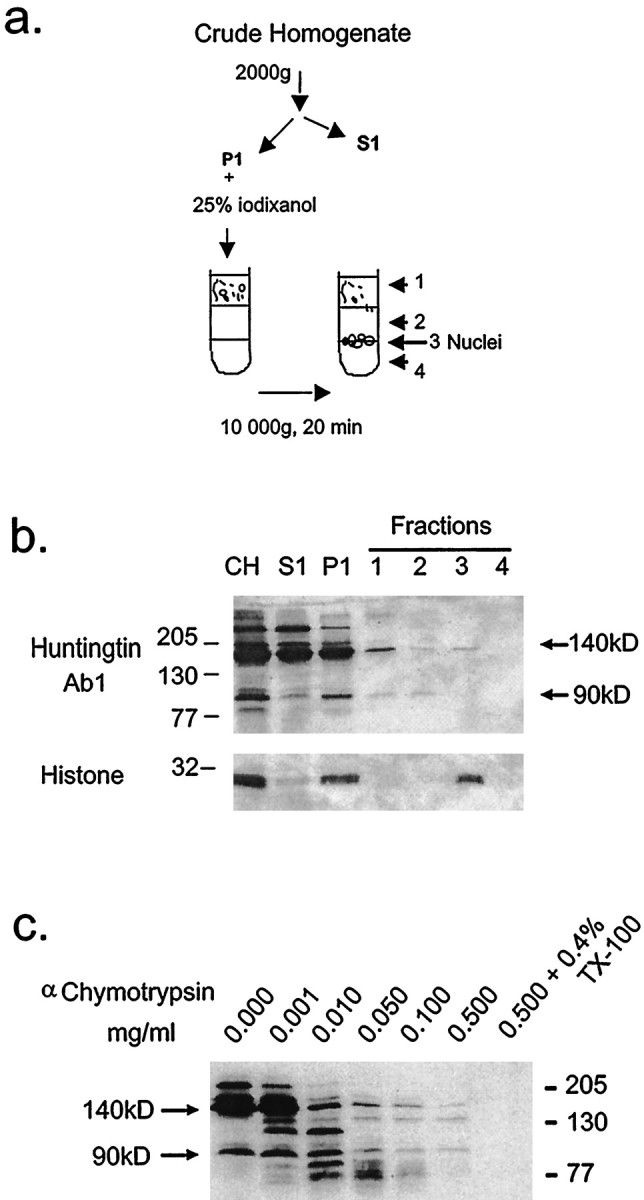
Biochemical analysis of P1 fraction and α-chymotrypsin digestion from cells transfected with FH3221-100. a, Scheme of nuclear isolation. The low speed pellet (P1) was fractionated on a discontinuous iodixanol density gradient as described in Methods and Materials. Fractions are as follows: CH, crude homogenate; S1, 2000 × gsupernatant; P1, 2000 × g pellet; fraction 1, 25% iodixanol step; fraction 2, 30% iodixanol step; fraction 3, 30%/35% interface; fraction 4, 35% iodixanol step. b, Western blots of fractions probed with anti-huntingtin antisera Ab1. Twenty microliters from each fraction was loaded per lane. Nuclear fraction (fraction 3) was identified using histone as a marker and contained the 140 kDa truncated huntingtin but not the N-terminal 90 kDa fragment. Apparent molecular weight is indicated in kilodaltons on theleft. c, Western blots of equal volumes of crude homogenates (CH). The crude homogenate was treated with varied amounts of α-chymotrypsin for 30 min on ice, stopped with 2 μl of 200 mm PMSF, and then analyzed by Western blot.
