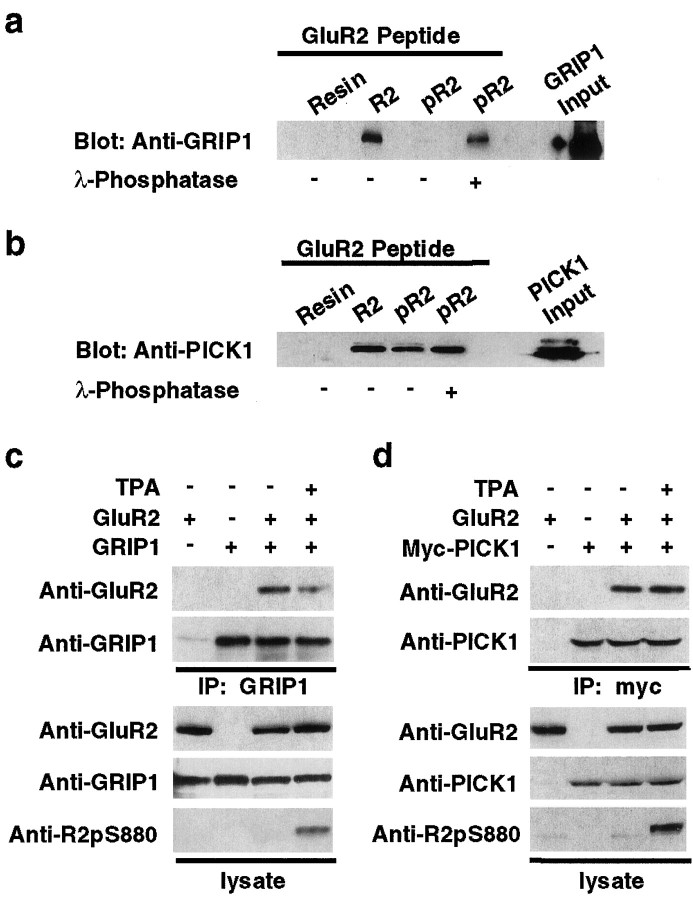
Fig. 3.
Ser880 phosphorylation of GluR2 C terminus differentially regulates the interaction of GluR2 with GRIP1 and PICK1.a, b, In vitro binding of GRIP1 (a) or PICK1 (b) with Ser880-phosphorylated GluR2 peptides. Extracts of HEK293T cells expressing GRIP1 or PICK1 were incubated with Ser880-phosphorylated R2 peptides (pR2) or unphosphorylated peptides (R2) immobilized on Affigel resins, and bound GRIP1 or PICK1 was detected by immunoblotting. a, GRIP1 did not interact with pR2 peptide, whereas phosphatase treatment of pR2 peptide recovered GRIP1 binding. b, PICK1 binds to both pR2 and R2 peptide. c, d, Coimmunoprecipitation of GluR2 with GRIP1 (c) or PICK1 (d) from transfected HEK293T cells, with or without 1 μm TPA treatment. Increase in Ser880 phosphorylation of GluR2 C terminus by PKC activation attenuated GRIP1 interaction with GluR2 (c) but had no effect on the binding affinity of PICK1 to GluR2 (d).