Abstract
To evaluate the cholinergic and dopaminergic neuronal interaction in the striatum, the effects of scopolamine, a muscarinic cholinergic antagonist, on the striatal dopaminergic system were evaluated multi-parametrically in the conscious monkey brain using high-resolution positron emission tomography in combination with microdialysis.l-3,4-Dihydroxyphenylalanine (l-[β-11C]DOPA) and 2β-carbomethoxy-3β-(4-fluorophenyl)tropane ([β-11C]CFT) were used to measure dopamine synthesis rate and dopamine transporter (DAT) availability, respectively. For assessment of dopamine D2 receptor binding in vivo, [11C]raclopride was applied because this labeled compound, which has relatively low affinity to dopamine D2 receptors, was hypothesized to be sensitive to the striatal synaptic dopamine concentration. Systemic administration of scopolamine at doses of 10 and 100 μg/kg dose-dependently increased both dopamine synthesis and DAT availability as measured byl-[β-11C]DOPA and [β-11C]CFT, respectively. Scopolamine decreased the binding of [11C]raclopride in a dose-dependent manner. Scopolamine induced no significant changes in dopamine concentration in the striatal extracellular fluid (ECF) as determined by microdialysis. However, scopolamine dose-dependently facilitated the striatal ECF dopamine induced by the DAT inhibitor GBR12909 at a dose of 0.5 mg/kg. Scatchard plot analysis in vivo of [11C]raclopride revealed that scopolamine reduced the apparent affinity of dopamine D2 receptors. These results suggested that the inhibition of muscarinic cholinergic neuronal activity modulates dopamine turnover in the striatum by simultaneous enhancement of the dynamics of dopamine synthesis and DAT availability, resulting in no significant changes in apparent “static” ECF dopamine level but showing a decrease in [11C]raclopride binding in vivoattributable to the reduction of affinity of dopamine D2 receptors.
Keywords: l-[β-11C]DOPA, [11C]raclopride, [β-11C]CFT, positron emission tomography, microdialysis, monkey brain
The neurotransmitter systems do not work in isolation, and they are anatomically and functionally integrated as a network directly (Hattori et al., 1976) or indirectly (Bunney and Aghajanian, 1976) through multisynaptic connections. Although neuropsychiatric and neurodegenerative diseases have been attributed to deficits within a single neurotransmitter system, disease progression might be related to the deficit of the initially affected system to modulate or be modulated by other neurotransmitters. The extrapyramidal motor system, for example, relies on a balance between dopamine and acetylcholine, and disruption in the balance results in motor abnormalities. Positron emission tomography (PET) can evaluate the functional responses of neurotransmitters to pharmacological manipulation, as well as the interactions between neuronal systems. PET has been used to assess the effects of endogenous dopamine (Innis et al., 1992; Dewey et al., 1993a; Carson et al., 1997), NMDA/glutamate (Smith et al., 1997), acetylcholine (Dewey et al., 1993b), serotonin (Dewey et al., 1995), and GABA (Dewey et al., 1992) on striatal [11C]raclopride binding (for review, see Laruelle, 2000). These reports suggested that the changes in striatal synaptic dopamine could be measured noninvasively by PET using [11C]raclopride, which has more moderate affinity for D2 receptors than [11C]N-methyl spiperone (NMSP) (Seeman et al., 1989; Young et al., 1991). The measurement is based on the principle that neurotransmitters might compete with a radiolabeled ligand on the receptors if the affinity of the ligand to the receptor is moderate. In fact, basic studies using rodents demonstrated that increases or decreases in dopamine concentration decreased or increased the in vivo binding of [3H]/[11C]raclopride, respectively. (Seeman et al., 1989; Inoue et al., 1991; Young et al., 1991; Ginovart et al., 1997). However, we demonstrated recently that the alternation of [11C]raclopride binding in vivo as measured by PET was not regulated simply by the apparent “static” dopamine level in the synapse, i.e., it represents the dynamic balance of release and reuptake rates of dopamine (Tsukada et al., 1999a, 2000a). Thus, in the conscious monkey brains in combination with animal PET and microdialysis, we showed that indirect dopamine modulators such as benztropine (a muscarinic cholinergic antagonist) and ketanserine (a 5-HT2antagonist) reduced [11C]raclopride binding in the striatum with much smaller degree of increase in synaptic dopamine than those induced by methamphetamine and GBR12909 (Tsukada et al., 1999a). In addition, ketamine, a noncompetitive NMDA receptor antagonist, reduced [11C]raclopride binding in the striatum without any significant change in the synaptic dopamine concentration (Tsukada et al., 2000a).
The aim of the present study was to explore the regulatory mechanisms between cholinergic and dopaminergic neuronal systems multi-parametrically using PET in combination withl-3,4-dihydroxyphenylalanine (l[β-11C]DOPA), [11C]raclopride, and 2β-carbomethoxy-3β-(4-fluorophenyl)tropane ([β-11C]CFT) in the conscious monkey brain. Scopolamine, a muscarinic cholinergic antagonist, was used as a modulator of the dopaminergic neuronal system instead of benztropine, which has a slight dopamine transporter (DAT) inhibitory effect in addition to its muscarinic cholinergic receptor inhibitory action (Coyle and Snyder, 1969). In vivo Scatchard plot analysis was applied to evaluate the effects of scopolamine on binding parameters of [11C]raclopride to dopamine D2 receptors. Microdialysis studies were conducted to assess the effects of scopolamine on dopamine concentrations in the striatal extracellular fluid (ECF).
MATERIALS AND METHODS
Animals and drugs. Young-adult male rhesus monkeys (Macaca mulatta; n = 4) weighing from 5.5 to 6.5 kg were used for the PET measurements. Monkeys were maintained and handled in accordance with recommendations of the United States National Institutes of Health and also the guidelines of the Central Research Laboratory, Hamamatsu Photonics. They were trained to sit on a chair twice a week over a period of >3 months. Magnetic resonance images (MRI) of all monkeys were obtained with a Toshiba MRT-50A/II (0.5 T) under pentobarbital anesthesia. The stereotactic coordinates of PET and MRI were adjusted based on the orbitomeatal (OM) line with a specially designed head holder (Takechi et al., 1994). At least 1 month before the PET study, an acrylic plate, with which monkey was fixed to a monkey chair, was attached to the head under pentobarbital anesthesia as described previously (Onoe et al., 1994).
Scopolamine hydrobromide was obtained from Kyorin Pharmaceutical Co. Ltd. (Tokyo, Japan). Precursors for labeling of [11C]raclopride and [β-11C]CFT were purchased from Research Biochemicals (Natick, MA). The enzymes forl[β-11C]DOPA synthesis, alanine racemase (EC 5.1.1.1.), d-amino acid oxidase (EC1.4.3.3.), and β-tyrosinase (EC 4.1.99.2), were purchased from Ikeda Food Research Co. Ltd. (Hiroshima, Japan).
Synthesis of [11C]-labeled compounds. Carbon-11 (11C) was produced by14N(p,α)11C nuclear reaction using a cyclotron (HM-18; Sumitomo Heavy Industry, Tokyo, Japan) at Hamamatsu Photonics PET center and obtained as [11C]CO2. [β-11C]CFT was labeled with11C by N-methylation of its nor-compound with [11C]methyl iodide prepared from [11C]CO2. [11C]Raclopride was synthesized byO-methylation of its precursor with [11C]methyl iodide. The radiochemical and chemical purities used here were greater than 98 and 99%, respectively, and the specific radioactivity ranged from 107 to 141 GBq/μmol for [β-11C]CFT and from 54.2 to 77.8 GBq/μmol for [11C]raclopride, respectively.
l-[β-11C]DOPA was synthesized using a combination of organic synthesis and multi-enzymatic procedures (Bjurling et al., 1990) using an automated synthesizer (Harada et al., 2000). The radiochemical and chemical purities of l-[β-11C]DOPA were better than 98 and 99%, respectively.
After analysis for identification, the solution was passed through a 0.22 μm pore size filter before intravenous administration to the monkey.
PET scan. Data were collected on a high-resolution PET scanner (SHR-7700; Hamamatsu Photonics, Hamamatsu, Japan) with a transaxial resolution of 2.6 mm full-width at half-maximum and a center-to-center distance of 3.6 mm (Watanabe et al., 1997). The PET camera allowed 31 slices for imaging to be recorded simultaneously.
After an overnight fast, animals were fixed to the monkey chair with stereotactic coordinates aligned parallel to the OM line. A cannula was implanted into the posterior tibial vein of the monkey for administration of [11C]-labeled ligands, and another cannula was put into the femoral artery of the other leg to obtain arterial blood samples for scans with [11C]raclopride and [β-11C]CFT.
During PET scans, heart rate, respiration rate, blood pressure, and body temperature were continuously monitored using a life monitoring system (Nihon Kohden, Tokyo, Japan). The levels of carbon dioxide (Paco2), blood oxygen (Pao2), and pH of arterial blood were measured with a Stat Profile blood gas analyzer (Nova Biochemical, Waltham, MA).
All four monkeys were subjected to PET scans withl-[β-11C]DOPA, [11C]raclopride, and [β-11C]CFT. Three PET scans with either [11C]-labeled compound were serially performed in the same animal in 1 d. At 30 min after administration of saline, a [11C]-labeled compound was injected through the posterior tibial vein cannula. For second and third scans, at 30 min after administration of scopolamine (10 or 100 μg/kg), the same [11C]-labeled compound was injected every 3 hr. Because of the very short half-life of11C (20.4 min), the radioisotope used in these studies, a time lag of at least 3 hr between scans provided sufficient time for decay of the radioactivity in the monkeys (∼ of injected dose), so that the level of radioactivity associated with the previous injection of [11C]-labeled compound would not interfere with the next scan.
PET scans with [11C]raclopride andl-[β-11C]DOPA were performed for 64 min with six time frames at 10 sec intervals, six time frames at 30 sec, 12 time frames at 1 min, followed by 16 time frames at 3 min. For [β-11C]CFT study, PET scans were performed with an additional nine time frames at 3 min.
Kinetic analysis of in vivo binding. Regions of interest (ROI), i.e., the striatum and cerebellum, were identified according to MR images of each monkey brain, and the time–activity curves ofl-[β-11C]DOPA, [11C]raclopride, and [β-11C]CFT in ROIs were obtained as described previously (Tsukada et al., 1999a,b, 2000a,b).
To measure the input function of [11C]raclopride and [β-11C]CFT to the brain, arterial blood samples were obtained every 8 sec from 10 to 66 sec, followed by 96, 156, 246, and 336 sec, and then 20, 30, 45, and 60 min after tracer injection. For [β-11C]CFT, additional samples were taken at 75 and 90 min. Blood samples were centrifuged to separate plasma and weighed, and their radioactivity was measured. For metabolite analysis, methanol was added to some plasma samples (sample/methanol, 1:1) obtained at 42 and 66 sec and 5.6, 10, 30, 45, 60, 75, and 90 min after tracer injection, followed by centrifugation. The obtained supernatants were developed with thin-layer chromatography (TLC) plates (AL SIL G/UV; Whatman, Kent, UK) with a mobile phase of ethylene dichloride/diethyl ether/ethanol/triethylamine, 20:20:1:1. The ratio of unmetabolized fraction was determined using a phosphoimaging plate (BAS-1500 MAC; Fuji Film Co. Ltd., Tokyo, Japan). Therf values of [11C]raclopride and [β-11C]CFT were 0.41 and 0.52, respectively. The input functions of unmetabolized [11C]raclopride and [β-11C]CFT were calculated using the data obtained by correction of the ratio of the unmetabolized fraction to total radioactivity.
For quantification of in vivo binding of [11C]raclopride and [β-11C]CFT, a kinetic three-compartment analysis method was applied as described previously (Huang et al., 1986). The time–activity curves of plasma and of each region were fitted to a three-compartment model with the least-square fitting method using the constrainedK1/k2ratio to the distribution volume in the cerebellum. The values of binding potential (BP) of [11C]raclopride and [β-11C]CFT were calculated by determining the ratio of the estimatedk3 value (association rate) to the estimated k4 value (dissociation rate) (Tsukada et al., 1999a,b, 2000a,b).
For quantification ofl-[β-11C]DOPA utilization rate constant in the striatum of the monkey brain, a graphical analysis method was applied to calculate dopamine synthesis rate (k3) as described previously (Tedroff et al., 1991; Tsukada et al., 1996b, 2000a,b).
Scatchard plot analysis. Saturation experiments were performed to examine the effects of scopolamine on in vivobinding parameters (Bmax andKd) of [11C]raclopride (Farde et al., 1989;Tsukada et al., 1996a). Thirty minutes after administration of scopolamine (10 and 100 μg/kg), [11C]raclopride was injected into monkeys under carrier-free conditions or together with various amounts (from 3 to 300 μg/kg) of carrier raclopride. The total radioligand concentration of [11C]raclopride in the cerebellum was used as an estimate of the free radioligand concentration (F) in the striatum. Specific binding (B) was defined as radioactivity in the striatum reduced by F. In the case of [11C]raclopride, the curve forB was fitted to a set of three exponential functions to determine the time point at which B reached a peak (Farde et al., 1989). The values for B and F at these time points were used in Scatchard analysis in which the ratio ofB/F was plotted against B (Scatchard, 1949). The apparent in vivo Bmax andKd values were analyzed using LIGAND software (Munson and Rodbard, 1980).
Microdialysis analysis. Microdialysis was performed in the conscious state in the same monkeys used for PET studies as described previously (Tsukada et al., 1999a,b, 2000a,b). A guide cannula was implanted (anterior, 21 mm; lateral, 3.0 mm) according to the individual MR images with reference to the stereotactic brain atlas ofSnider and Lee (1961), during the procedure for attachment of the acrylic plate. A microdialysis probe with a membrane region 250 μm in diameter and 3 mm in length (Eicom A-I-25–03; Eicom, Tokyo, Japan) was inserted into the striatal region (17.0 mm below the dura matter) of the monkey brain via the guide cannula. The probe was initially perfused with Krebs'–Henseleit solution (in mm: 118.5 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.25 CaCl2, 25.0 NaHCO3, and 5.6 glucose, pH 7.4) at a rate of 10 μl/min to remove dopamine overflow from the damaged tissue. The perfusion rate was decreased to 5 μl/min 2 hr after insertion of the probe, 75 μl samples were collected every 15 min, and the content of dopamine was measured by HPLC with electrochemical detection. To verify the exact positioning of the probe, 5 μl of China ink was injected via the guide cannula at the end of the experiments. Animals were anesthetized with sodium pentobarbital and decapitated. The brains were quickly removed, coronal sections were cut on a cryostat, and the location of the probe implantation site was determined visually.
The averaged data obtained from 0 to 120 min before administration of saline or scopolamine were used as “ baseline” data. Saline or scopolamine (10 and 100 μg/kg) was administered 120 min after the start of sampling. GBR12909 at a dose of 0.5 mg/kg was administered 30 min after saline or scopolamine (10 and 100 μg/kg). The striatal ECF dopamine level was expressed as percentage of baseline.
Statistical analysis. Results are expressed as means ± SD. Comparison between conditions was performed using the paired, two-tailed Student's t test, and a probability level ofp < 0.05 was considered statistically significant.
RESULTS
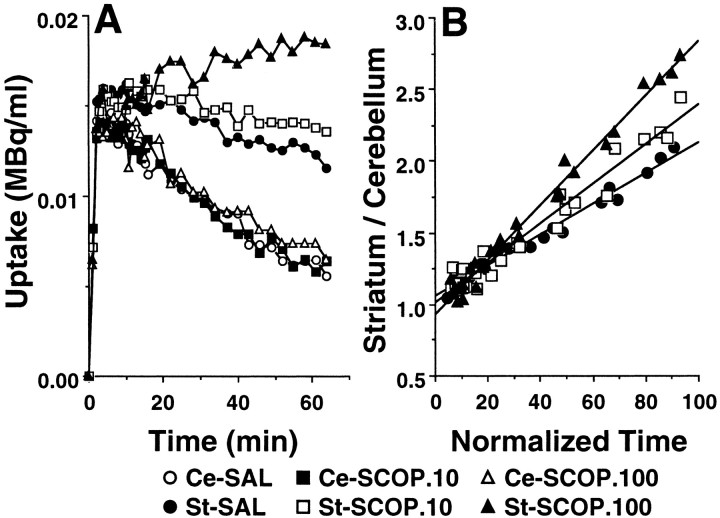
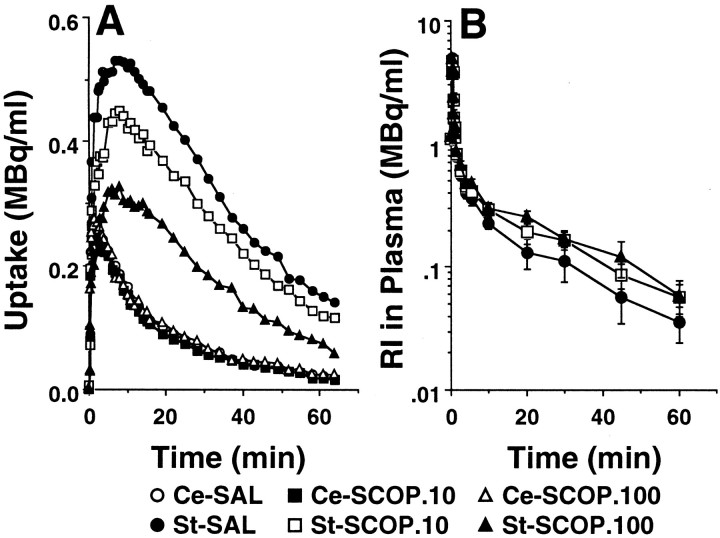
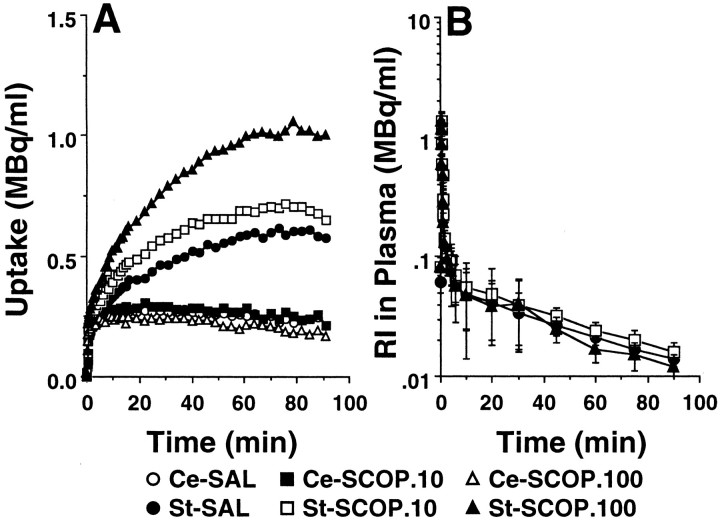
Under the control conditions with saline administration, the summated PET images from 37 to 64 min after injection and the time–activity curves indicated high uptake ofl-[β-11C]DOPA (Fig.1A), [11C]raclopride (Fig.2A), and [β-11C]CFT (Fig.3A) in the striatum, and low uptake in the cerebellum of the conscious monkey brain. The striatal radioactivity associated withl-[β-11C]DOPA reached a peak 5 min after injection and remained at this elevated level to the end of the scan (Fig. 1A). The maximum accumulation of radioactivity in the striatum occurred ∼10 min after injection of [11C]raclopride and decreased gradually thereafter (Fig. 2A). The time–activity curve of [β-11C]CFT in the striatum increased with time during the experimental period (Fig.3A). In the cerebellum, the time–activity curves of these labeled compounds showed peak values within 5 min after injection, followed by gradual decreases with time (Figs. 1A,2A, 3A).
Fig. 1.
Effects of scopolamine on the time–activity curves of l-[β-11C]DOPA in the brain (A) and the k3 value (B). Saline or scopolamine (10 or 100 μg/kg) was administered 30 min beforel-[β-11C]DOPA injection. PET scan was started immediately after tracer injection, and image data were collected for 64 min. A, ROIs were identified according to MRI of the same animal. The radioactivity in each striatum (St) and cerebellum (Ce) were plotted against time after tracer injection. B, The time course of changes in radioactivity in the striatum as a ratio to that in the cerebellum was expressed as a function of the normalized integral of each cerebellar radioactivity. The slope of the calculated regression line represents k3 value.
Fig. 2.
Effects of scopolamine on the time–activity curves of [11C]raclopride in the brain (A) and in the arterial plasma (B). Saline or scopolamine (10 or 100 μg/kg) was administered 30 min before [11C]raclopride injection. PET scan was started immediately after tracer injection, and image data were collected for 64 min. A, ROIs were identified according to MRI of the same animal. The radioactivity in each striatum (St) and cerebellum (Ce) were plotted against time after tracer injection. B, Time-activity curve of unmetabolized [11C]raclopride. Unmetabolized [11C]raclopride was calculated by correction of relative to total radioactivity with the ratio of the unmetabolized fraction at each time point.
Fig. 3.
Effects of scopolamine on the time–activity curves of [β-11C]CFT in the brain (A) and in the arterial plasma (B). Saline or scopolamine (10 or 100 μg/kg) was administered 30 min before [β-11C]CFT injection. PET scan was started immediately after tracer injection, and image data were collected for 91 min. A, ROIs were identified according to MRI of the same animal. The radioactivity in each striatum (St) and cerebellum (Ce) were plotted against time after tracer injection. B, Time–activity curve of unmetabolized [β-11C]CFT. Unmetabolized [β-11C]CFT was calculated by correction of relative to total radioactivity with the ratio of the unmetabolized fraction at each time point.
In plasma, the curves of total radioactivity associated with [11C]raclopride (Fig.2B) and [β-11C]CFT (Fig. 3B) showed peaks ∼30 sec after slow bolus intravenous injection and declined rapidly thereafter. Metabolite analysis by TLC and a phosphoimaging system indicated that [11C]raclopride and [β-11C]CFT were gradually metabolized to very polar metabolites, which remained at the origin, and the ratios of radioactivity in unmetabolized labeled compounds to the in total (unmetabolized plus metabolized) were 0.72 and 0.22 at 60 min after injection, respectively (data not shown). The input functions of unmetabolized [11C]-labeled compounds were calculated using the data obtained by correction of total radioactivity relative to the metabolic ratio (data not shown). In general, the input functions of [11C]raclopride and [β-11C]CFT into the brain were not significantly affected by administration of scopolamine (Figs.2B, 3B).
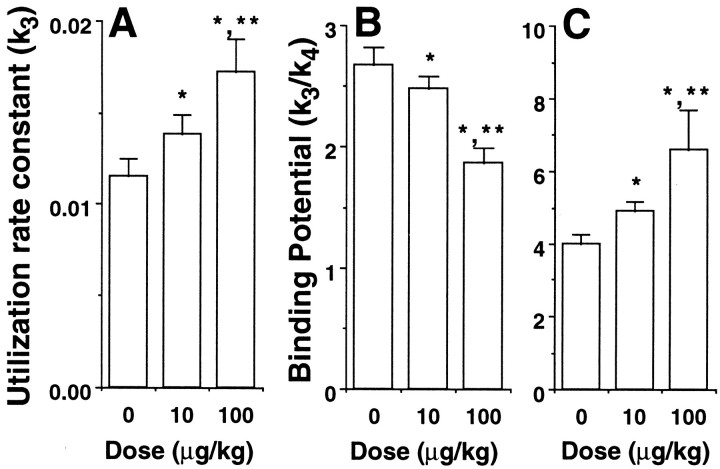
Systemic intravenous administration of scopolamine at doses of 10 and 100 μg/kg caused dose-dependent increases in the uptake ofl-[β-11C]DOPA in the striatum with no significant changes in the radioactivity curves in the cerebellum (Fig. 1A). These alterations in the striatal kinetics ofl-[β-11C]DOPA caused dose-dependent enhancement of the kinetic values of dopamine synthesis rate (k3), as calculated using the time–activity curve of the cerebellum as the input function (Figs. 1B,4A).
Fig. 4.
Effects of scopolamine on dopamine synthesis rate, D2 receptor binding, and transporter availability, as measured with l-[β-11C]DOPA (A), [11C]raclopride (B), and [β-11C]CFT (C), respectively. A, The time course of changes in radioactivity in the striatum (St) as a ratio of that in the cerebellum (Ce) was expressed as a function of the normalized integral of each cerebellar radioactivity. The values of k3 are represented by the slope of the calculated regression line shown in Figure 1B. B, C, The time–activity curves of unmetabolized [11C]raclopride and [β-11C]CFT in arterial plasma, shown in Figures 2B and 3B, were used as input functions into the brain, and the three-compartment model was fitted to the time–activity curve of specific binding in the striatum. The binding potential was calculated as the ratio of the association rate (k3) to the dissociation rate (k4). Data are expressed as means ± SD for four animals per treatment condition. *p < 0.05 versus respective saline control (dose of 0). **p < 0.05 versus respective scopolamine at a dose of 10 μg/kg.
The administration of scopolamine (10 and 100 μg/kg) resulted in a dose-dependent reduction of [11C]raclopride uptake in the striatum, with no significant changes in those in the cerebellum or arterial plasma (Fig. 2A,B). These alterations in the striatal kinetics of [11C]raclopride caused a dose-dependent decrease in the binding potential (BP =k3/k4) as calculated using each plasma time–activity curve as an input function (Fig. 4B).
The administration of scopolamine (10 and 100 μg/kg) caused a dose-dependent increase in the uptake of [β-11C]CFT in the striatum with no significant changes in the radioactivity curves of arterial plasma (Fig. 3A,B), indicating the dose-dependent increase in the BP of [β-11C]CFT as shown in Figure4C.
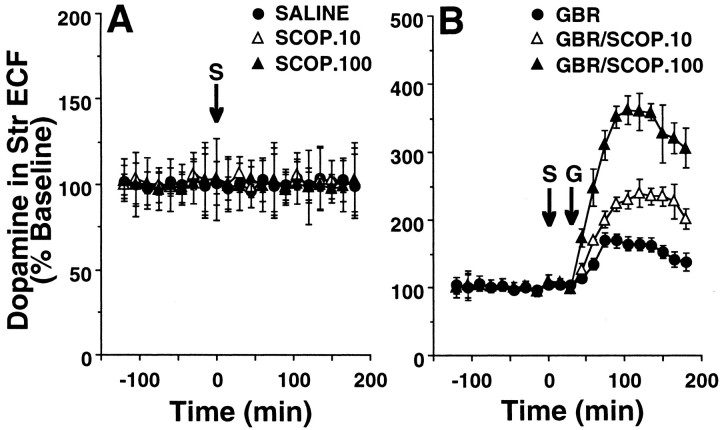
As shown in Figure 5, the effects of scopolamine (10 and 100 μg/kg) on dopamine level in the striatal ECF were evaluated by microdialysis in the monkey brain. Microdialysis was performed simultaneously with PET scans of [11C]raclopride. The baseline level of dopamine was 6.5 ± 1.9 fmol/μl (n = 4) in the striatum of conscious monkeys. Administration of scopolamine at any dose used here resulted in no significant changes in the striatal ECF dopamine level (Fig. 5A). When GBR12909, a specific DAT inhibitor, was administered at a dose of 0.5 mg/kg in saline-treated animals, it significantly increased dopamine level in the striatal ECF of the monkey brain (Fig. 5B). Interestingly, scopolamine preadministered 30 min before the administration of GBR12909 at the same dose as used in the saline condition further facilitated the GBR12909-induced striatal ECF dopamine enhancement in a dose-dependent manner (Fig. 5B).
Fig. 5.
Effects of scopolamine (A) and/or GBR12909 (B) on dopamine concentration in the striatal ECF of the monkey brain. A microdialysis probe was inserted into the striatal region via the guide cannula. The probe was perfused with Krebs'–Henseleit solution (pH 7.4) at a rate of 5 μl/min. Samples were collected every 15 min, and the content of dopamine was measured by HPLC with electrochemical detection. The averaged data obtained from 0 to 120 min without any infusion were used as baseline data. Saline or scopolamine at doses of 10 or 100 μg/kg was administered 120 min after the start of sampling (arrow with S). GBR12909 at a dose of 0.5 mg/kg was administered 30 min after saline or scopolamine administration (arrow with G). The striatal ECF dopamine level was expressed as percentage of baseline.
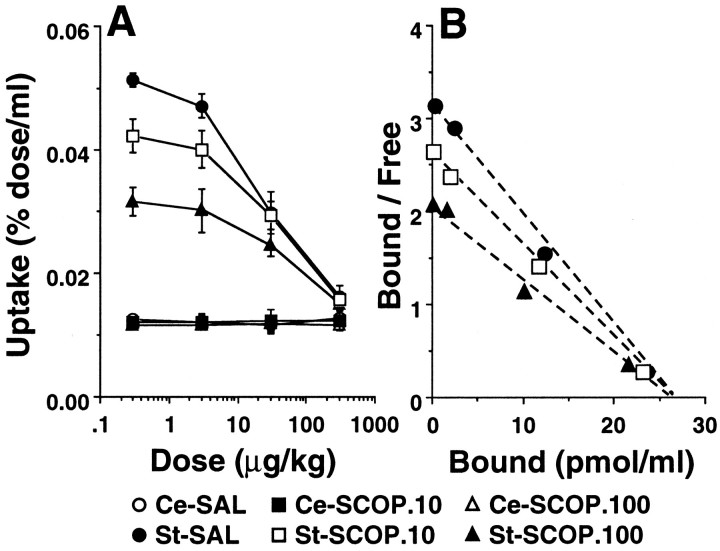
To clarify the mechanism(s) by which scopolamine modulates dopamine D2 availability in vivo, [11C]raclopride was injected into monkeys together with various amounts (from 3 to 300 μg/kg) of unlabeled carrier ligand raclopride. [11C]Raclopride with carrier ligand was injected 30 min after the administration of scopolamine (10 and 100 μg/kg). The addition of increasing amounts of unlabeled carrier ligand dose-dependently reduced the amounts of bound radiolabeled ligand (Fig. 6A). In these studies, a significant decrease in radioactivity of bound [11C]raclopride in the striatum over the time span of the PET study was found, and the results were similar in both saline- and scopolamine-treated groups. In contrast, in the cerebellum, no changes were observed in the amount of radioactivity of [11C]raclopride over the range of amount of unlabeled carrier added in both cases (Fig.6A). The free radioligand concentration (F) in the striatum was assumed to be comparable with the radioligand concentration in the cerebellum. The specific binding (B) in the striatum was calculated by subtracting the radioligand concentration measured in the cerebellum from total binding in the striatum. For Scatchard analysis of the binding of [11C]raclopride in vivo, equilibrium values for B and F were obtained when the B value was maximum. The maximum accumulation occurred between 12 and 15 min after injection of [11C]raclopride. The Scatchard plot revealed a linear curve for [11C]raclopride in saline-treated, as well as scopolamine-treated, monkeys (Fig. 6B). The administration of scopolamine resulted in dose-dependent reduction of the slopes of the curves determined with [11C]raclopride, suggesting the alteration of the affinity (1/Kd) of D2 receptors (Fig. 6B). In contrast, scopolamine did not affect the intercept with thex-axis in [11C]raclopride, which provided the maximum number of binding sites (Bmax) of D2receptors (Fig. 6B).
Fig. 6.
Saturation studies (A) and Scatchard plot analysis (B) of in vivo binding of [11C]raclopride in the monkey brain. Monkeys were administered saline or scopolamine (10 or 100 μg/kg, i.v.) 30 min before injection of [11C]raclopride under carrier-free conditions or with various doses of carrier ligand ranging from 3 to 300 μg/kg. A, The radioactivities in striatum and cerebellum at 12–15 min after tracer injection were expressed as percentage of dose per milliliter. Data are expressed as means ± SD for four animals per treatment group. Radioactivity in the striatum (open symbols) or cerebellum (filled symbols). B, The total radioligand concentration in the cerebellum was used as the free radioligand concentration (Free) in the striatum. Specific binding (Bound) was defined as radioactivity in the striatum reduced with Free. The average values (n = 4 for each points) for Boundand Free were used in a Scatchard analysis in which the ratios Bound/Free were plotted againstBound.
DISCUSSION
This is the first study to demonstrate the effects of muscarinic cholinergic modulation on the striatal dopamine neuronal activity by simultaneous multi-parametric assessment of dopamine synthesis, D2 receptor binding and DAT availability as measured by PET in the same nonhuman primates in the conscious state.
We reported previously that benztropine, a muscarinic cholinergic antagonist with slight DAT inhibitory activity, induced the reduction of [11C]raclopride binding in the conscious monkey brain as measured by PET (Tsukada et al., 1999a). These observations were consistent with the previous report of inhibition of [11C]raclopride binding by cholinergic blockade induced by scopolamine in anesthetized baboons (Dewey et al., 1993b). Although these authors speculated that the reduced [11C]raclopride binding was attributable to the increase in synaptic dopamine level via neuronal interactions, it did not demonstrate a clear relationship between the doses administered and the reduction in magnitude of [11C]raclopride binding (Dewey et al., 1993b). The lack of microdialysis data as measured in the same animals used in the PET study impaired interpretation of the previous data. The change in synaptic dopamine level does not always represent a reasonable explanation for the alterations of dopamine D2 receptor availability in vivo as measured by PET with [11C]raclopride. As we have demonstrated previously, although administration of benztropine-induced elevation of dopamine concentration in the striatal ECF as measured by microdialysis, the magnitude of the elevation of dopamine level by benztropine was much lower and of shorter duration than those evoked by direct dopamine enhancers, such as GBR12909 and methamphetamine (Tsukada et al., 1999a). However, benztropine induced significant reduction of [11C]raclopride in the striatum of conscious monkeys to a similar extent as that induced by the direct dopamine enhances (Tsukada et al., 1999a). Furthermore, benztropine also decreased [18F]NMSP (Dewey et al., 1990), which was expected to be more stable against alterations of dopamine concentration than [11C]raclopride because of its 10 times higher affinity to dopamine D2 receptors. As shown in the present study, scopolamine did not alter the apparent static dopamine concentration in the striatal ECF; however, scopolamine reduced thein vivo binding of [11C]raclopride. Amphetamine reduced thein vivo binding of both [3H]/[11C]raclopride (Dewey et al., 1991, 1993a; Young et al., 1991) and [3H]/[18F]NMSP (Dewey et al., 1991; Logan et al., 1991; Young et al., 1991). Isoflurane has been reported to increase synaptic dopamine levels (Opacka-Juffry et al., 1991); however, it decreased the in vivo binding of [11C]raclopride to a much lesser extent than that of [11C]NMSP (Kobayashi et al., 1995). Subanesthetic doses of ketamine, a noncompetitive antagonist of NMDA receptors, decreased the striatal binding of [11C]raclopride in human subjects, suggesting that ketamine increased the striatal dopamine concentration by ketamine (Smith et al., 1997). However, the results of previous microdialysis studies demonstrated that NMDA antagonists [ketamine and (+)-5-methyl-10,11-dihydro-5H-dibenzo(a,d)cyclohepten-5,10-imine hydrogen maleate (MK-801)] increased (Moghaddam et al., 1990;French 1994; Verma and Moghaddam, 1996), decreased (Kashihara et al., 1990), or did not change (Bacopoulos et al., 1979; Koshikawa et al., 1988; Onoe et al., 1994; Tsukada et al., 2000a) dopamine concentration in the striatum. It is of interest that MK-801 significantly increased stereotypic behavior but did not affect the striatal dopamine concentration (Weihmuller et al., 1991). Our previous studies indicated that ketamine paradoxically altered dopamine D2receptor availability as measured by [11C]raclopride (increased binding) and [11C]NMSP (decreased binding) without any apparent change in dopamine concentration in the striatal ECF (Onoe et al., 1994; Tsukada et al., 2000a). Modulation using reserpine, which depletes endogenous dopamine, induced the increased binding of [3H]/[11C]raclopride (Inoue et al., 1991; Ginovart et al., 1997) but the decreased binding of [3H]NMSP (Inoue et al., 1991) (for review, see Laruelle, 2000). Interestingly, [11C]raclopride binding in vivo was also markedly reduced in the human striatum during video game playing with hand movement, and the degree of reduction showed a reverse correlation with the performance of the game, showing at most 50% reduction of binding (Koepp et al., 1998). In this study, the reduction of [11C]raclopride was simply attributed to the increased dopamine in the striatal synaptic cleft. However, in the animal experiment with conscious monkeys, 50% reduction of [11C]raclopride required the administration of methamphetamine at 1 mg/kg or more, which corresponds to an almost 100-fold higher dose than that used by drug abusers daily (Tsukada et al., 1999a). Together, the alteration of affinity or availability change of receptor sites induced by the neuronal interactions should also be taken into account for the alteration of dopamine D2 receptor availabilityin vivo as measured by PET with [11C]raclopride, as well as [11C]NMSP. In fact, the present results obtained by Scatchard plot analysis in vivo demonstrated that cholinergic modulation elicited alteration of the apparent affinity (1/Kd) of dopamine D2 receptors, without any alteration of the apparent maximum numbers of binding sites (Bmax) of dopamine D2 receptors, resulting in the reduced binding potential BP =k3/k4=Bmax/Kdof [11C]raclopride in vivo in the monkey brain as measured by PET.
It was hypothesized previously that the altered affinity of dopamine D2 receptors measured by [11C]raclopride accounted for the apparent static concentration of endogenous dopamine in the synaptic cleft (Farde et al., 1995). Although a reduction in affinity of [11C]raclopride was consistent with a competitive model between endogenous dopamine and [11C]raclopride binding to receptors, the present results did not support the previous hypothesis, because similar analytical procedures revealed that scopolamine administration resulted in decreased affinity of dopamine D2receptors as measured by [11C]raclopride without any changes in apparent static dopamine concentration in the striatal ECF as measured by microdialysis. The present results obtained from the assessments of dopamine synthesis and DAT availability provide the important insight into these mechanisms. Systemic administration of scopolamine produced the simultaneous and dose-dependent enhancement of dopamine synthesis and DAT availability as measured byl-[β-11C]DOPA and [β-11C]CFT, respectively. Previous studies demonstrated that the utilization rate constant (k3) ofl-[β-11C]DOPA was increased by enhancement of the activity of tyrosine hydroxylase (EC 1.14.16.2) (Tsukada et al., 1996b), which is the rate-limiting enzyme in the synthesis of catecholamines (Nagatsu et al., 1964), accompanied with increased dopamine release into the synaptic cleft (Tsukada et al., 1994b). As observed in the present study, administration of scopolamine increased the utilization rate constant (k3) ofl-[β-11C]DOPA in a dose-dependent manner, suggesting that dopamine synthesis was enhanced in addition to dopamine release into the synaptic cleft of the striatum. However, microdialysis assay indicated no significant increase in dopamine concentration in the striatal ECF in the present study. One possible explanation for this discrepancy is that the change in dopamine concentration in ECF measured by microdialysis does not reflect the “true” dopamine release into the synaptic cleft as suggested previously using voltammetry (Kuhr et al., 1984; Kuhr and Wightman, 1986; May, 1988; Grace, 1993). This is unlikely however, because our previous study demonstrated that the same microdialysis assay could detect the enhancement of dopamine synthesis in the neurons (Tsukada et al., 1994a,b), the facilitation of dopamine release by methamphetamine (Tsukada et al., 1999a), and also the increase in the synaptic dopamine level induced by the inhibition of DAT by cocaine and GBR12909 (Tsukada et al., 1999a,b). Then the resulting increase in dopamine concentration in the striatal ECF was confirmed by measurements of “cold” endogenous dopamine (Tsukada et al., 1994a, 1999a,b) and [11C]-labeled “hot” dopamine converted froml-[β-11C]DOPA (Tsukada et al., 1994b). The precise mechanisms of enhanced DAT availability by scopolamine remain unclear yet. Because of the slow kinetics of [β-11C]CFT in the brain, its delivery is often affected by the change in regional cerebral blood flow (rCBF); that is, the increased rCBF might result in the enhanced uptake of [β-11C]CFT as observed in the present study. However, changes in rCBF might not account for the enhanced DAT availability by scopolamine, because our previous result demonstrated that the administration of scopolamine at the doses of 10 and 100 μg/kg decreased, not increased, rCBF in the conscious monkey brain (Tsukada et al., 1997). Some compensatory mechanisms with negative feedback system might be involved in this enhanced DAT availability for the regulation of increased dopamine release. Our recent results also revealed that ketamine infusion dose-dependently decreased [11C]raclopride binding with no significant changes in dopamine concentration in the striatal ECF as observed in the case of scopolamine, and also that ketamine increased both dopamine synthesis and DAT availability as measured byl-[β-11C]DOPA and [β-11C]CFT, respectively (Tsukada et al., 2000a). Microdialysis using the DAT inhibitor GBR12909 demonstrated that preadministration of scopolamine further facilitated the increase in striatal ECF dopamine level induced by DAT inhibition. The present results further indicated the facilitation of dopamine turnover by scopolamine and also strongly supported the usefulness of the combined use ofl-[β-11C]DOPA and [β-11C]CFT for the assessment of dopamine turnover. Together, these results suggested that the modulation of muscarinic cholinergic, as well as glutamatergic, neuronal activities altered dopamine turnover in the striatum by simultaneous enhancement of the dynamics of dopamine synthesis and DAT availability to the same extent, resulting in no apparent marked changes in ECF dopamine concentration as measured by microdialysis.
In conclusion, the present results revealed that scopolamine reduced dopamine D2 receptor binding in vivoby increasing dopamine turnover rate, not by elevating apparent static dopamine concentration in the synaptic cleft, resulting in the altered receptor affinity or availability. That is, the regulatory mechanism of dopamine neuronal transmission might be explained by the “rate” theory defined as the dynamics of dopamine binding to receptors and synaptic turnover of dopamine, not by the conventional “occupancy” theory. These results further support our hypothesis that alteration of the binding of radiolabeled ligands in vivo as measured by PET might not simply be modulated by the apparent static synaptic concentration of dopamine (Tsukada et al., 1999a, 2000a). These observations will be important for research and diagnosis of neuropsychiatric and neurodegenerative diseases using the functional imaging modalities of PET and single photon emission tomography with labeled compounds.
Footnotes
This work was supported in part by Special Coordination Funds for Promoting Science and Technology of the Science and Technology Agency of the Japanese Government. We are grateful Kengo Sato and Dai Fukumoto for their excellent technical assistance.
Correspondence should be addressed to Dr. Hideo Tsukada, Central Research Laboratory, Hamamatsu Photonics K.K., 5000 Hirakuchi, Hamakita, Shizuoka 434-8601, Japan. E-mail: tsukada@crl.hpk.co.jp.
REFERENCES
- 1.Bacopoulos NG, Redmond DE, Roth RH. Serotonin and dopamine metabolism in brain regions and cerebrospinal fluid of a primate species: effects of ketamine and fluphenazine. J Neurochem. 1979;32:1215–1218. doi: 10.1111/j.1471-4159.1979.tb11048.x. [DOI] [PubMed] [Google Scholar]
- 2.Bjurling P, Watanabe Y, Oka S, Nagasawa T, Yamada H, Långström B. Multienzymatic synthesis of β-11C-labelled l-tyrosine and l-DOPA. Acta Chem Scand. 1990;44:183–188. [Google Scholar]
- 3.Bunney BS, Aghajanian GK. Dopaminergic influence in the basal ganglia: evidence for striatonigral feedback regulation. In: Yahr M, editor. The basal ganglia. Raven; New York: 1976. pp. 249–267. [PubMed] [Google Scholar]
- 4.Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B, Der MG, Picker D, Eckelman WC. Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab. 1997;17:437–447. doi: 10.1097/00004647-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Coyle JT, Snyder SH. Antiparkinsonian drugs: inhibition of dopamine uptake in the corpus striatum as a possible mechanism of action. Science. 1969;166:899–901. doi: 10.1126/science.166.3907.899. [DOI] [PubMed] [Google Scholar]
- 6.Dewey SL, Brodie JD, Fowler JS, MacGregor RR, Schlyer DJ, King P, Alexoff D, Volkow ND, Shiue C-Y, Wolf AP, Bendriem B. Positron emission tomography (PET) studies of dopamine/cholinergic interactions in the baboon brain. Synapse. 1990;6:321–327. doi: 10.1002/syn.890060403. [DOI] [PubMed] [Google Scholar]
- 7.Dewey SL, Logan J, Wolf AP, Brodie JD, Angrist B, Fowler JS, Volkow ND. Amphetamine induced decreases in (18F)-N-methylspiperidol binding in the baboon brain using positron emission tomography (PET). Synapse. 1991;7:324–427. doi: 10.1002/syn.890070409. [DOI] [PubMed] [Google Scholar]
- 8.Dewey SL, Smith GS, Logan J, Brodie JD, Yu DW, Ferrieri RA, King PT, MacGregor RR, Martin TP, Wolf AP, Volkow ND, Fowler JS, Meller E. GABAergic inhibition of endogenous dopamine release measured in vivo with 11C-raclopride and positron emission tomography. J Neurosci. 1992;12:3773–3780. doi: 10.1523/JNEUROSCI.12-10-03773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey SL, Smith GS, Logan J, Brodie JD, Fowler JS, Wolf AP. Striatal binding of the PET ligand 11C-raclopride is altered by drugs that modify synaptic dopamine levels. Synapse. 1993a;13:350–356. doi: 10.1002/syn.890130407. [DOI] [PubMed] [Google Scholar]
- 10.Dewey SL, Smith GS, Logan J, Simkowitz P, Brodie JD, Volkow ND, Fowler JS, Wolf AP. Effects of central cholinergic blockade on striatal dopamine release measured with positron emission tomography (PET) in normal human subjects. Proc Natl Acad Sci USA. 1993b;90:11816–11820. doi: 10.1073/pnas.90.24.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dewey SL, Smith GS, Logan J, Alexoff D, Ding Y-S, King P, Pappas N, Brodie JD, Ashby CR. Serotonergic modulation of striatal dopamine measured with positron emission tomography (PET) and in vivo microdialysis. J Neurosci. 1995;15:821–829. doi: 10.1523/JNEUROSCI.15-01-00821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farde L, Eriksson L, Blomquist G, Halldin C. Kinetic analysis of central [11C]raclopride binding to D2-dopamine receptors studied by PET: a comparison to the equilibrium analysis. J Cere Blood Flow Metab. 1989;9:696–708. doi: 10.1038/jcbfm.1989.98. [DOI] [PubMed] [Google Scholar]
- 13.Farde L, Hall H, Pauli S, Halldin C. Variability in D2-dopamine receptor density and affinity: a PET study with [11C]raclopride in man. Synapse. 1995;20:200–208. doi: 10.1002/syn.890200303. [DOI] [PubMed] [Google Scholar]
- 14.French E. Phencyclidine and the midbrain dopamine system: electrophysiology and behavior. Neurotoxicol Teratol. 1994;16:355–362. doi: 10.1016/0892-0362(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 15.Ginovart N, Farde L, Halldin C, Swahn CG. Effect of reserpine-induced depletion of synaptic dopamine on [11C]raclopride binding to D2-dopamine receptors in the monkey brain. Synapse. 1997;25:321–325. doi: 10.1002/(SICI)1098-2396(199704)25:4<321::AID-SYN2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 16.Grace AA. Cortical regulation of subcortical systems and its possible relevance to schizophrenia. J Neural Transm. 1993;91:111–134. doi: 10.1007/BF01245228. [DOI] [PubMed] [Google Scholar]
- 17.Harada N, Nishiyama S, Sato K, Tsukada H. Development of an automated synthesis apparatus for l-[3-11C]labeled aromatic amino acids. Appl Radiat Isot. 2000;52:845–850. doi: 10.1016/s0969-8043(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 18.Hattori T, Singh VK, McGeer PL, McGeer EG. Immunohistochemical localization of choline acetyltransferase containing neostriatal neurons and their relationship with dopaminergic synapse. Brain Res. 1976;102:164–173. doi: 10.1016/0006-8993(76)90583-7. [DOI] [PubMed] [Google Scholar]
- 19.Huang SH, Barrio J, Phelps M. Neuroreceptor assay with positron emission tomography; equilibrium versus dynamic approach. J Cereb Blood Flow Metab. 1986;6:515–521. doi: 10.1038/jcbfm.1986.96. [DOI] [PubMed] [Google Scholar]
- 20.Innis RB, Malison RT, Al-Tikrite M, Hoffer PB, Sybirska EH, Seibyl JP, Zoghbi SS, Baldwin RM, Laruelle M, Smith EO, Charney DS, Heninger G, Elsworth JD, Roth RH. Amphetamine-stimulated dopamine release compete in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- 21.Inoue O, Kobayashi K, Tsukada H, Itoh T, Långström B. Difference in in vivo receptor binding between [3H]N-methylspiperone and [3H]raclopride in reserpine-treated mouse brain. J Neural Transm. 1991;85:1–10. doi: 10.1007/BF01244652. [DOI] [PubMed] [Google Scholar]
- 22.Kashihara K, Hamamura T, Okumura K, Otsuki S. Effect of MK-801 on endogenous dopamine release in vivo. Brain Res. 1990;528:80–82. doi: 10.1016/0006-8993(90)90197-j. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Inoue O, Watanabe Y, Onoe H, Långström B. Difference in response of D2 receptor binding between 11C-N-methylspiperone and 11C-raclopride against anesthetics in rhesus monkey brain. J Neural Transm. 1995;100:147–151. doi: 10.1007/BF01271537. [DOI] [PubMed] [Google Scholar]
- 24.Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- 25.Koshikawa N, Tomiyama K, Omiya K, Kobayashi M. Ketamine anesthesia has no effect on striatal dopamine metabolism in rats. Brain Res. 1988;444:394–396. doi: 10.1016/0006-8993(88)90954-7. [DOI] [PubMed] [Google Scholar]
- 26.Kuhr WG, Wightman RM. Real-time measurement of dopamine release in rat brain. Brain Res. 1986;381:168–71. doi: 10.1016/0006-8993(86)90707-9. [DOI] [PubMed] [Google Scholar]
- 27.Kuhr WG, Ewing AG, Caudill WL, Wightman RM. Monitoring the stimulated release of dopamine with in vivo voltammetry. I. Characterization of the response observed in the caudate nucleus of the rat. J Neurochem. 1984;43:560–569. doi: 10.1111/j.1471-4159.1984.tb00935.x. [DOI] [PubMed] [Google Scholar]
- 28.Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Logan J, Dewey SL, Wolf AP, Fowler J, Brodie JD, Angrist B, Volkow ND, Gatley SJ. Effects of endogenous dopamine on measures of [F-18]NMSP binding in the basal ganglia: Comparison of stimulations and experimental results from PET studies in baboons. Synapse. 1991;9:195–207. doi: 10.1002/syn.890090306. [DOI] [PubMed] [Google Scholar]
- 30.May LJ. Differentiation of dopamine overflow and uptake processes in the extracellular fluid of the rat caudate nucleus with fast-scan in vivo voltammetry. J Neurochem. 1988;51:1060–1069. doi: 10.1111/j.1471-4159.1988.tb03069.x. [DOI] [PubMed] [Google Scholar]
- 31.Moghaddam B, Gruen R, Roth R, Bunney B, Adams R. Effects of l-glutamate on the release of striatal dopamine: in vivo dialysis and electrochemical studies. Brain Res. 1990;518:55–60. doi: 10.1016/0006-8993(90)90953-9. [DOI] [PubMed] [Google Scholar]
- 32.Munson PJ, Rodbard D. LIGAND: a versatile computerized approach for the characterization of ligand binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 33.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase: the initial steps in norepinephrine biosynthesis. J Biol Chem. 1964;239:2910–2920. [PubMed] [Google Scholar]
- 34.Onoe H, Inoue O, Suzuki K, Tsukada H, Ito T, Magata N, Watanabe Y. Ketamine increases the striatal N-11C-methylspiperone binding in vivo: positron emission tomography study using conscious rhesus monkey. Brain Res. 1994;663:191–198. doi: 10.1016/0006-8993(94)91263-7. [DOI] [PubMed] [Google Scholar]
- 35.Opacka-Juffry J, Ahier RG, Cremer JE. Nomifensine-induced increase in extracellular striatal dopamine is enhanced by isoflurane anesthesia. Synapse. 1991;7:169–171. doi: 10.1002/syn.890070210. [DOI] [PubMed] [Google Scholar]
- 36.Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 37.Seeman P, Guan HC, Niznik HB. Endogenous dopamine lowers the dopamine D2 receptor density as measured by [3H]raclopride: implications for positron emission tomography of the human brain. Synapse. 1989;3:96–97. doi: 10.1002/syn.890030113. [DOI] [PubMed] [Google Scholar]
- 38.Smith GS, Schloesser R, Brodie JD, Dewey SL, Logan J, Vitkun SA, Simkowitz P, Hurley A, Cooper T, Volkow ND, Cancro R. Glutamate modulation of dopamine measured in vivo with positron emission tomography (PET) and 11C-raclopride in normal human subjects. Neuropsychopharmacology. 1997;18:18–25. doi: 10.1016/S0893-133X(97)00092-4. [DOI] [PubMed] [Google Scholar]
- 39.Snider R, Lee JC. In a stereotactic atlas of the monkey brain (Macaca mulatta). Chicago UP; Chicago: 1961. [Google Scholar]
- 40.Takechi H, Onoe H, Imamura K, Onoe K, Kakiuchi T, Nishiyama S, Yoshikawa E, Mori S, Kosugi T, Okada H, Tsukada H, Watanabe Y. Brain activation study by use of positron emission tomography in unanesthetized monkey. Neurosci Lett. 1994;182:279–282. doi: 10.1016/0304-3940(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 41.Tedroff J, Aquilonius SM, Hartvig P, Lundqvist H, Bjurling P, Långström B. Estimation of regional cerebral utilization of [11C]- l-3,4-dihydroxyphenylalanine (DOPA) in the primate by positron emission tomography. Acta Neural Scand. 1991;85:166–173. doi: 10.1111/j.1600-0404.1992.tb04021.x. [DOI] [PubMed] [Google Scholar]
- 42.Tsukada H, Lindner KJ, Hartvig P, Långström B. Effect of 6R- l-erythro-5,6,7,8-tetrahydrobiopterin on the extracellular levels of dopamine and serotonin in the rat striatum: a microdialysis study with tyrosine or tryptophan infusion. Brain Res. 1994a;635:59–67. doi: 10.1016/0006-8993(94)91423-0. [DOI] [PubMed] [Google Scholar]
- 43.Tsukada H, Lindner KJ, Hartvig P, Tani Y, Bjurling P, Kihlberg T, Westerberg G, Watanabe Y, Långström B. Effect of 6R- l-erythro-5,6,7,8-tetrahydrobiopterin on in vivol-[β-11C]DOPA turnover in the rat striatum with infusion of l-tyrosine. J Neural Transm Gen Sect. 1994b;95:1–15. doi: 10.1007/BF01283026. [DOI] [PubMed] [Google Scholar]
- 44.Tsukada H, Kreuter J, Maggos CE, Unterwald EM, Kakiuchi T, Nishiyama S, Futatsubashi M, Kreek MJ. Effects of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: an in vivo study using positron emission tomography. J Neurosci. 1996a;16:7670–7677. doi: 10.1523/JNEUROSCI.16-23-07670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsukada H, Lindner KJ, Hartvig P, Tani Y, Valtysson J, Bjurling P, Kihlberg T, Westerberg G, Watanabe Y, Långström B. Effect of 6R- l-erythro-5,6,7,8-tetrahydrobiopterin and infusion of l-tyrosine on the in vivol-[β-11C]DOPA disposition in the monkey brain. Brain Res. 1996b;713:92–98. doi: 10.1016/0006-8993(95)01489-6. [DOI] [PubMed] [Google Scholar]
- 46.Tsukada H, Kakiuchi T, Ando I, Shizuno H, Nakanishi S, Ouchi Y. Regulation of cerebral blood flow response to somatosensory stimulation through the cholinergic system: a PET study in unanesthetized monkey brain. Brain Res. 1997;749:10–17. doi: 10.1016/s0006-8993(96)01028-1. [DOI] [PubMed] [Google Scholar]
- 47.Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N. Is synaptic dopamine concentration the exclusive factor which alters the in vivo binding of [11C]raclopride? PET studies combined with microdialysis in conscious monkeys. Brain Res. 1999a;841:160–169. doi: 10.1016/s0006-8993(99)01834-x. [DOI] [PubMed] [Google Scholar]
- 48.Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N, Nakanishi S. Isoflurane anesthesia enhances the inhibitory effects of cocaine and GBR12909 on dopamine transporter: PET studies in combination with microdialysis in the monkey brain. Brain Res. 1999b;849:85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- 49.Tsukada H, Harada N, Nishiyama S, Ohba H, Sato K, Fukumoto D, Kakiuchi T. Ketamine decreased striatal [11C]raclopride binding with no alterations in static dopamine concentrations in the striatal extracellular fluid in the monkey brain: multi-parametric PET studies combined with microdialysis analysis. Synapse. 2000a;37:95–103. doi: 10.1002/1098-2396(200008)37:2<95::AID-SYN3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 50.Tsukada H, Harada N, Nishiyama S, Ohba H, Kakiuchi T. Dose response and duration effects of acute administrations of cocaine and GBR12909 on dopamine synthesis and transporter in the conscious monkey brain: PET studies combined with microdialysis. Brain Res. 2000b;860:141–148. doi: 10.1016/s0006-8993(00)02057-6. [DOI] [PubMed] [Google Scholar]
- 51.Verma A, Moghaddam B. NMDA receptor antagonists impair prefrontal cortex function as assessed via spatial delayed alteration performance in rats: modulation by dopamine. J Neurosci. 1996;16:373–379. doi: 10.1523/JNEUROSCI.16-01-00373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe M, Okada H, Shimizu K, Omura T, Yoshikawa E, Kosugi T, Mori S, Yamashita T. A high resolution animal PET scanner using compact PS-PMT detectors. IEEE Trans Nucl Sci. 1997;44:1277–1282. [Google Scholar]
- 53.Weihmuller FB, O'Dell SJ, Cole BN, Marshall JF. MK-801 attenuates the dopamine-releasing but not the behavioral effects of methanphetamine: an in vivo microdialysis study. Brain Res. 1991;549:230–235. doi: 10.1016/0006-8993(91)90462-5. [DOI] [PubMed] [Google Scholar]
- 54.Young LT, Wong DF, Goldman S, Minkin E, Chen C, Matsumura K, Scheffel U, Wagner HN. Effects of endogenous dopamine on kinetics of [3H]N-methylspiperone and [3H]raclopride binding in the rat brain. Synapse. 1991;9:188–194. doi: 10.1002/syn.890090305. [DOI] [PubMed] [Google Scholar]