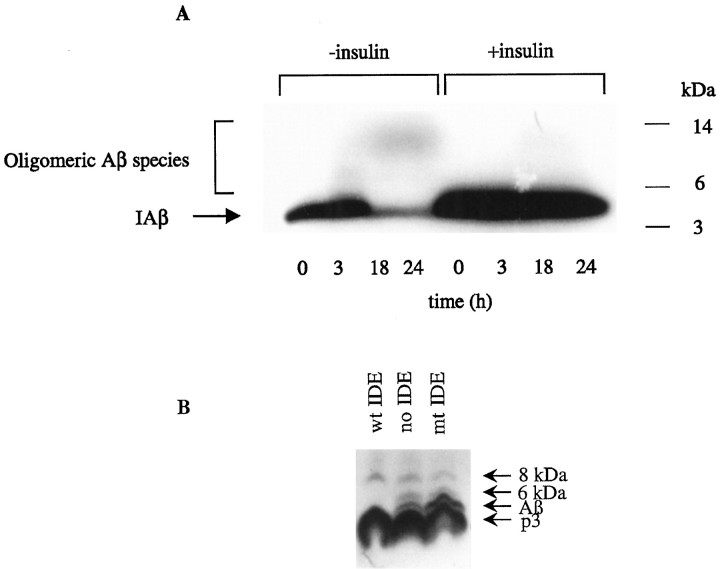
Fig. 4.
IDE stimulates the oligomerization of iodinated synthetic Aβ but not naturally secreted Aβ in neuronal cultures.A, Primary mixed cortical cultures were conditioned in serum-free medium overnight and incubated with IAβ (300 pm) for up to 24 hr in the presence or absence of insulin (10 μm). Aliquots of the reaction mixtures were removed at the times indicated and characterized by 10–20% Tris/Tricine gel fluorography. Note that the loss of Aβ monomer and the formation of small amounts of SDS-stable higher molecular weight species are almost abolished by insulin. B, 7PA2 CHO cells were labeled with [35S]Met for 12 hr in the absence (no IDE) or presence of 100 ng of purified recombinant active IDE (wt IDE) or mutant (E111Q) IDE (mt IDE). CM were collected, immunoprecipitated with the Aβ antibody R1282, and assayed by SDS-PAGE and fluorography. Note that the 6 kDa species has been shown to be a modified form of Aβ monomer that migrates anomalously in SDS-PAGE gels; it always follows the behavior of the 4 kDa conventional Aβ monomer, in contrast to that of the 8 kDa Aβ dimer (Podlisny et al., 1998) (D. Walsh, R. Wong, and D. J. Selkoe, unpublished observations).