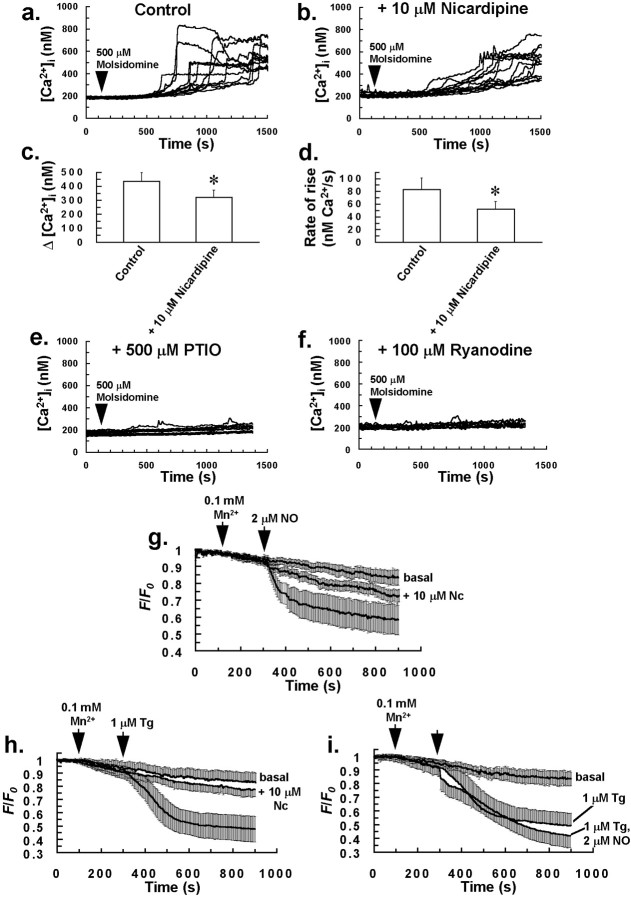
Fig. 4.
NO induces Ca2+ influx in glia that is nicardipine-sensitive. The rate of rise and magnitude of increases in [Ca2+]i induced by bolus addition of the NO donor molsidomine to the incubation bath (a) were reduced by pretreating cells with 10 μm nicardipine for 5 min (b).c, Graph derived from the experiments ofa and b showing a reduction in Δ[Ca2+]i for cells that responded to 500 μm molsidomine, after pretreatment of cells with 10 μm nicardipine. d, Graph derived from the experiments of a and b showing a reduction in the rate of rise of [Ca2+]i of the final burst phase, for cells that demonstrated an increase in [Ca2+]i to 500 μmmolsidomine, after pretreatment of cells with 10 μmnicardipine. Bars in c and d indicate the means of at least four separate estimations, and error bars indicate SD (*p < 0.05 vs control value; Student'st test for unpaired observations). Molsidomine-induced elevations in [Ca2+]i were completely abrogated by pretreating cells with 500 μm PTIO for 5 min (e) or 100 μm ryanodine for 30 min (f) before molsidomine application (arrow). Traces in a,b, e, and f are from 12 individual cells and are representative of at least four separate experiments for each of the above treatments. Bolus application of 2 μm aqueous NO (g) or 1 μm Tg (h) to cells resulted in Mn2+ quench of cytosolic fura-2 fluorescence (365 nm excitation) that was abrogated by pretreating cells with 10 μm nicardipine (Nc; center traces) for 5 min. There was no significant additive effect on the Mn2+ quench of cytosolic fura-2 fluorescence with a combined application of NO and Tg, compared to Tg alone (i), suggesting that NO acts on the same Ca2+ influx pathway as Tg in glial cells. All traces in g–i represent the mean normalized fluorescence intensity of at least 200 cells from four separate experiments for each of the above treatments. Error bars indicate SD. For measurements of basal Mn2+ quench of cytosolic fura-2 fluorescence (top traces) in g–i, no drugs were added to cells.