Fig. 1.
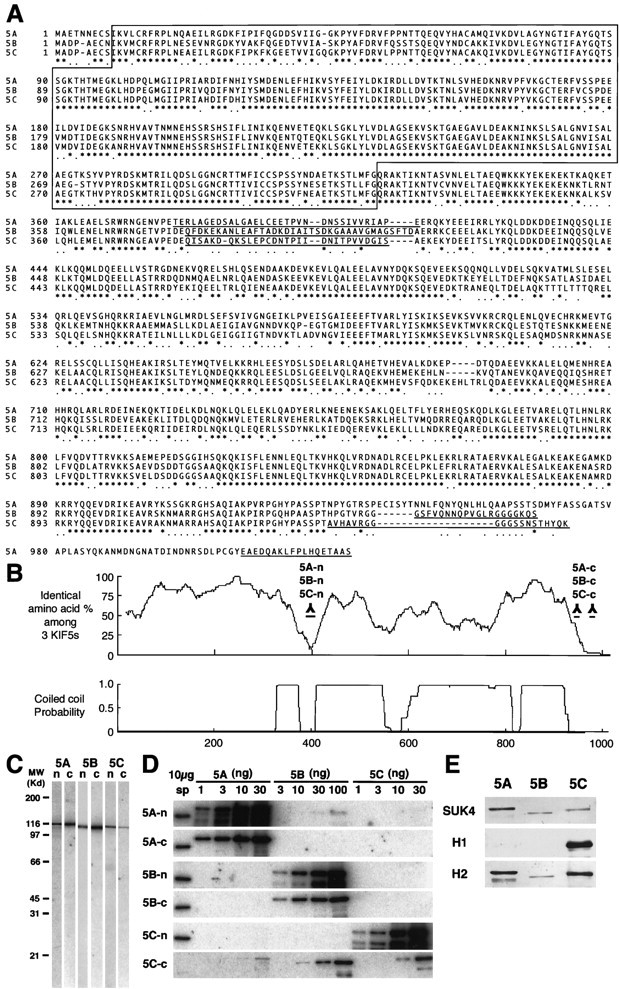
Alignment of the three KIF5s and antibody characterization. A, Alignment of the sequences of the three KIF5s. Amino acids are numbered in theleft margin. Asterisks represent the identical amino acids among the three KIF5s, and dotsshow the same residues between two KIF5s. The motor domain, located in the N-terminal region, is boxed. The amino acid sequences used for the antibody generation areunderlined. B, Homology among the three KIF5s and coiled-coil probability of KIF5C. There were two regions that showed very low similarity among the three KIF5s, with a small coiled-coil probability. We used these sequences for generating antibodies specific for each of the KIF5s (5A-n, 5B-n, 5C-n, 5A-c, 5B-c, and 5C-c). C, Immunoblot of anti-KIF5 antibodies (5A-n, 5B-n, 5C-n, 5A-c, 5B-c, and 5C-c). Ten micrograms of crude extract from an adult mouse brain were loaded on eachlane. D, Examination of the specificity of the six antibodies using recombinant KIF5 proteins. Filters transferred with 10 μg of crude extract from adult mouse spinal cord (sp) and various amounts of recombinant KIF5 proteins (KIF5A and KIF5C, 1, 3, 10, and 30 ng; KIF5B, 3, 10, 30, and 100 ng) were used. 5A-n showed weak cross-reactivity for KIF5B. 5A-c, 5B-n, 5B-c, and 5C-n did not cross-react with the other members of KIF5. 5C-c was not a specific antibody. E, Immunoblot analysis to characterize the widely used monoclonal anti-kinesin antibodies (SUK4, H1, and H2), using equal amount of recombinant KIF5 proteins (10 ng for SUK4 and H2; 30 ng for H1). SUK4 predominantly stained KIF5A; H1 detected only KIF5C; H2 preferred KIF5A and KIF5C to KIF5B. H1 did not recognize KIF5A or KIF5B, even when 100 ng of proteins were loaded (data not shown).
