Scheme 2.
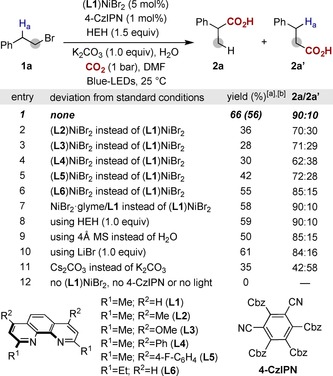
Optimization of the reaction conditions. 1 a (0.20 mmol), (L1)NiBr2 (5 mol %), 4‐CzIPN (1 mol %), HEH (1.5 equiv), K2CO3 (1.0 equiv), H2O (5.0 equiv), CO2 (1 bar), Blue‐LEDs in DMF (0.1 m) at 25 °C for 5 h. [a] Yields determined by NMR using 1,3,5‐trimethoxybenzene as standard. [b] Isolated yield, average of two independent runs. 4‐CzIPN: 2,4,5,6‐tetra(carbazol‐9‐yl)‐isophthalonitrile; DMF=N,N‐dimethylformamide; Cbz=carbazole; HEH=diethyl 1,4‐dihydro‐2,6‐dimethyl‐3,5‐pyridinedicarboxylate.
