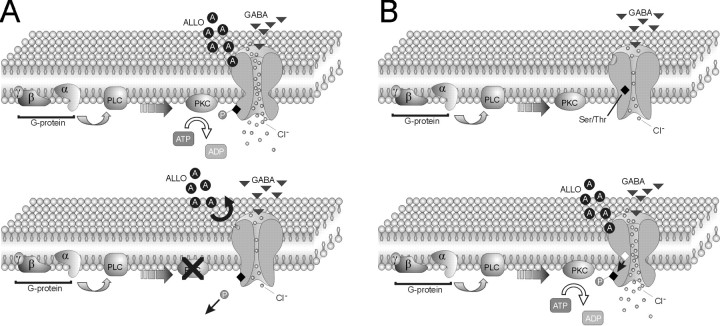
Fig. 6.
Models of G-protein and protein kinase dependence of GABAA receptor modulation by allopregnanolone. Our findings suggest that phosphorylation of GABAA receptors is necessary for the neurosteroid regulation of synaptic GABA currents, although enhancing G-protein and PKC activity alone is not sufficient to mimic the effect of the neurosteroid. This codependence on receptor phosphorylation and steroid presence can be explained by the following two models. A,Top, The first model is one in which the GABAA receptor is constitutively phosphorylated (P), allowing the neurosteroid (ALLO; A) to bind and increase channel opening and chloride flux. Bottom, Blocking G-protein and/or PKC activity leads to dephosphorylation of the channel, causing a conformational change in the receptor protein that prevents the neurosteroid from binding. B,Top, In the second model, in the absence of the neurosteroid the phosphorylation site (Ser/Thr) in the GABAA receptor is hidden from the constitutively active PKC. Bottom, After allopregnanolone binding, a conformational change in the GABAA receptor occurs, and the Ser/Thr residue(s) (open diamond) becomes exposed for phosphorylation by PKC, increasing channel opening and chloride influx.PLC, Phospholipase C.