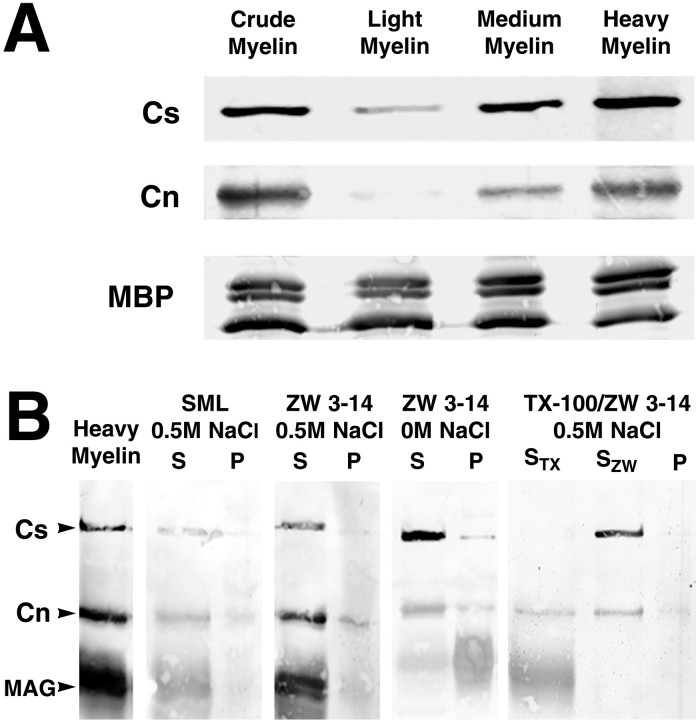
Fig. 4.
Caspr and contactin cofractionate and coextract from myelin fractions. Western blot analysis of Caspr and contactin in myelin fractions and detergent extracts is shown. A, Crude myelin preparations from rat brain homogenates are separated further into light, medium, and heavy myelin fractions. The heavy myelin fraction, which contains axonal and glial membranes, is enriched in paranodal junctions, as indicated by the increased concentration of Caspr (Cs). Contactin (Cn) is also enriched in this fraction, whereas myelin basic protein (MBP) is found in all of the fractions. Each lane was loaded with 50 μg of total protein. B, Detergent extracts were prepared from heavy myelin membranes, and the supernatant (S) and insoluble pellet (P) were evaluated for the presence of Caspr and contactin. The distribution of myelin-associated glycoprotein (MAG), a protein not found at the paranodal junctions, was also examined. Caspr and contactin can be solubilized from a heavy myelin preparation by sucrose monolaurate (SML) and Zwittergent 3-14 (ZW 3-14) with or without 0.5m NaCl. In the absence of salt Caspr and contactin, but very little MAG, are extracted by ZW 3-14. In contrast, Triton X-100 solubilizes most of the MAG (STX), whereas Caspr and contactin are mainly insoluble. These two proteins could then be coextracted by ZW 3-14 from the Triton-insoluble pellet (SZW).