Figure 3.
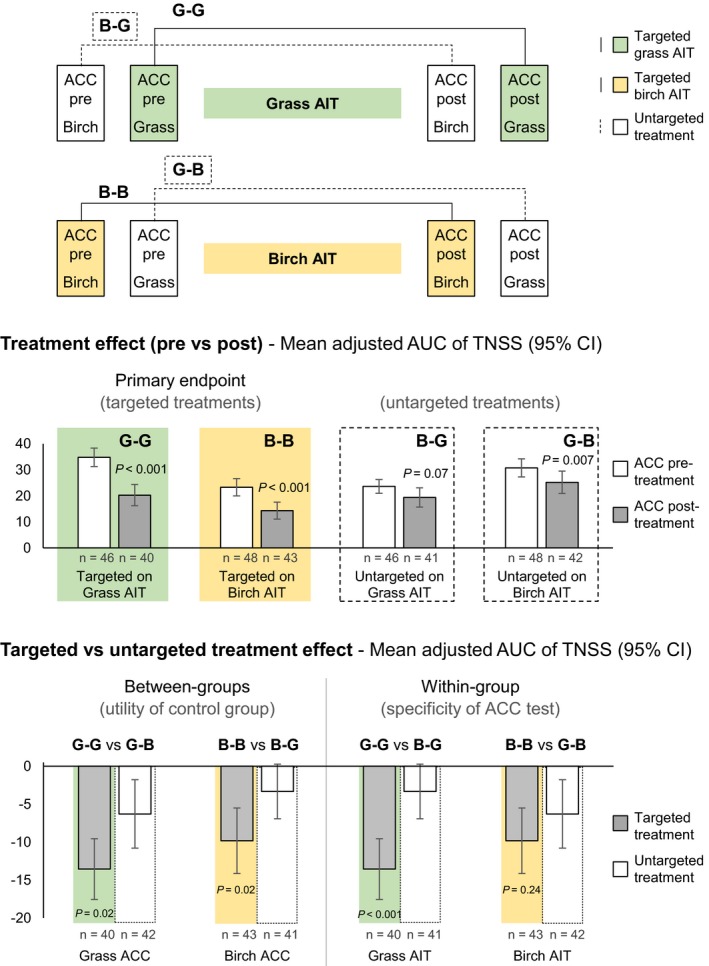
Overview of targeted and untargeted effects of allergen immunotherapy on total nasal symptom score (full analysis set). ACC, allergen challenge chamber; AIT, allergen immunotherapy; AUC, area under the curve; B‐B, birch‐on‐birch treatment effect (targeted); B‐G, birch‐on‐grass treatment effect (untargeted); CI, confidence interval; G‐B, grass‐on‐birch treatment effect (untargeted); G‐G, grass‐on‐grass treatment effect (targeted); n, number of patients with data; TNSS, total nasal symptom score. Between‐groups comparisons evaluate the utility of an “active allergen placebo”; within‐group comparisons evaluate the specificity of the ACC measurement. P‐value from 2‐sided 1‐sample t test. Results for the per‐protocol set analysis were similar to full analysis set for all primary and secondary endpoints
