ABSTRACT
The cytoskeleton protein α-fodrin plays a major role in maintaining structural stability of membranes. It was also identified as part of the brain γ-tubulin ring complex, the major microtubule nucleator. Here, we investigated the requirement of α-fodrin for microtubule spindle assembly during mitotic progression. We found that α-fodrin depletion results in abnormal mitosis with uncongressed chromosomes, leading to prolonged activation of the spindle assembly checkpoint and a severe mitotic delay. Further, α-fodrin repression led to the formation of shortened spindles with unstable kinetochore-microtubule attachments. We also found that the mitotic kinesin CENP-E had reduced levels at kinetochores to likely account for the chromosome misalignment defects in α-fodrin-depleted cells. Importantly, we showed these cells to exhibit reduced levels of detyrosinated α-tubulin, which primarily drives CENP-E localization. Since proper microtubule dynamics and chromosome alignment are required for completion of normal mitosis, this study reveals an unforeseen role of α-fodrin in regulating mitotic progression. Future studies on these lines of observations should reveal important mechanistic insight for fodrin’s involvement in cancer.
KEYWORDS: α-Fodrin, mitosis, spindle, SAC, CENP-E, detyrosinated tubulin
Graphical Abstract
Introduction
Non-erythroid spectrin or fodrin is a cytoskeleton protein belonging to the spectrin family characterized by spectrin repeats. Fodrin is a heterodimer composed of alpha and beta subunits encoded by SPTAN1 and SPTBN1, respectively [1,2]. Fodrin subunits are known to interact with other cytoskeletal components such as actin, protein 4.1 and ankyrin, thus providing a structural framework and stability to the cells. α-Fodrin has multiple functional motifs such as the calpain and caspase cleavage site, SH3, calcium and calmodulin binding domains, while β-fodrin contains the actin binding domain, ankyrin and protein 4.1 binding sites [3,4]. Though their major function has been attributed to the structural stability of the plasma membrane, the presence of the aforementioned motifs suggests a possible contribution to multiple cellular mechanisms such as signal transduction and apoptosis [5–7]. Recent studies have disclosed unsuspected functions of non-erythroid spectrin. Loss of α-fodrin results in increased mutation frequency as it is reported to interact with multiple DNA repair proteins [8,9]. Moreover, loss of α-fodrin leads to defective chromosomal inter-strand crosslink repair [10]. Studies have also reported the involvement of α-fodrin in cell cycle regulation since its depletion leads to G1 arrest [11]. Furthermore, we reported the presence of α-fodrin as a component from brain derived γ-TuRCs [12], and as a centrosomal component in brain-specific cell lines [13], suggesting that it might be involved in microtubule organization. However, comparative analysis with other tissues indicated this to be a neuronal-specific interaction. Since fodrin is known to bind to microtubules [14] and it is thought to have a role in the cell cycle [11], we asked whether α-fodrin may play a role in microtubule spindle organization and mitotic progression. In this study, we found a significant increase in chromosome alignment and spindle defects with delayed mitotic progression upon α-fodrin depletion. Analysis of mitotic motor proteins revealed that CENP-E, primarily required for chromosome alignment, was decreased at kinetochores upon α-fodrin depletion. This correlates with reduced levels of detyrosinated α-tubulin in α-fodrin deficient cells. Our studies thus disclose an unforeseen role of α-fodrin in spindle organization and mitotic progression.
Materials
PIPES, HEPES, PMSF, DTT, DAPI, Reversine, Protease inhibitor cocktails were from Sigma-Aldrich (St. Louis, Missouri, USA). OptiMEM, DMEM and Antibiotic-Antimycotic mix were from GIBCO BRL (Waltham, Massachusetts, USA). Lipofectamine 2000 was from Invitrogen (Carlsbad, California, USA). ECL reagent was from Thermo Scientific (Waltham, Massachusetts, USA). FBS was purchased from PAN Biotech, Aidenbach, Germany. Vectashield was procured from Vector Labs, Burlingame, California, USA. α-fodrin shRNA, control shRNA were procured from Origene Technologies (Rockville, Maryland, USA) and Santa Cruz (Dallas, Texas, USA). All other chemicals were of reagent grade.
Antibodies
Primary Antibodies: Antibodies against α-fodrin (ab2558)(WB: 1:1000, ab133320 IF 1:400) detyrosinated α-tubulin (ab48389) (WB 1:1000, IF:1:500), CENP-E (ab5093)(IF:1:500), BubR1 (ab4637) (IF:1:500) were procured from Abcam (Cambridge, United Kingdom).
Gamma-tubulin (PA5-34815)(WB:1:1000, IF:1:500), Actin (MA5-15739)(WB:1:2500), GAPDH (MA5-15738)(WB:1:5000) antibodies were from Thermo Scientific, USA and α-Tubulin (#3873)(WB:1:2000, IF:1:500) antibody was from Cell Signaling Technologies (Danvers, Massachusetts, USA). CREST antibody (90C-CS1058)(IF:1:500) was from Fitzgerald Industries International Inc (Acton, Massachusetts, USA).
Secondary antibodies: Anti-Mouse HRP (A-3673)(1:5000), Anti-Rabbit HRP (A-6154)(1:5000) were procured from Sigma-Aldrich (St. Louis, Missouri, USA). Anti-Mouse Alexa Fluor 488 (A-21202)(1:1000), Anti-Mouse Alexa Fluor 568 (A-10037) (1:1000), Anti-Rabbit Alexa Fluor 488 (A-11008)(1:1000), Anti-Rabbit Alexa Fluor 568 (A-11011)(1:1000) and Anti-human Alexa Fluor 647 (A-21445)(1:500) were from Molecular Probes (Eugene, Oregon, USA).
Cell lines
U-251 MG (Glioblastoma), HeLa (Cervical Cancer, CCL-2) and IMR 32 (Neuroblastoma, CCL-127) cell lines were obtained from ATCC, USA. The cell lines were checked for authentication by STR analysis in Rajiv Gandhi Centre for Biotechnology. Routine mycoplasma contamination check was also carried out.
Maintenance of cell lines
The cell lines used in the study were maintained in DMEM containing 10% FBS and 1X antibiotic mixture (100 units/mL of penicillin, 100 units/mL of streptomycin and 0.25 μg/mL of Amphotericin B). The cells were incubated at 37°C in a CO2 incubator in humid condition containing 5% CO2. From the reference stock, frozen stocks of cells were made within passage 3 and stored in liquid nitrogen. For experiments, cells were used within 3 months after revival.
shRNA transfection
Cells were seeded on coverslips in 35 mm dishes and transfection was carried out using Lipofectamine 2000 according to manufacturer’s protocol (Invitrogen). After 96 h, the cells were fixed for imaging or pelleted for western blotting analysis. In case of live cell imaging, after 72 h, the cells were incubated with DMEM without phenol red containing 10% FBS, 1% sodium pyruvate and 350 ng/mL of doxycycline for 24 h and then imaged. For downregulation experiments, single shRNA (5'ACAATCACCATGAGGAGAACATCTCTTCA 3') targeted against α-fodrin (Origene Technologies, USA) or combination of three different shRNA targeted against α-fodrin (Santa Cruz, USA) with the sense sequences 5'CGAGAGGAACUGAUUACAATT3', 5'GAGAGGAACUGAUUACAAAtt3', 5' CCACUGAACUGAAAGGAAUtt 3', were used and compared with scrambled shRNA.
Preparation of cell lysates
Cells were harvested by scraping them and washing with 1 X PBS. They were then lysed with Phospholysis buffer (1% NP-40, 10% Glycerol, 137 mM NaCl, 20 mM Tris-Cl pH 7.4, 20 mM NaF, 1 mM Sodium pyrophosphate, 1 mM Sodium orthovanadate, 1% Triton X 100) containing protease inhibitor cocktail for 45 min on ice. Subsequently, the cells were pelleted at 13,000 rpm for 5 min. The supernatant was collected and protein concentration was estimated by Bradford’s method. To the supernatant, 5X protein sample buffer was added and protein samples were heated for 5 min at 95°C in a heating block. The protein samples were then subjected to western blotting and developed using enhanced chemiluminescence.
Cell permeabilization buffer for detecting kinetochore-MT attachment
Cells were subjected to permeabilization buffer (100 mM PIPES, 1 mM MgCl2, 0.1 mM CaCl2 and 0.1% Triton X-100) for 2 min and then fixed in 4% paraformaldehyde in the same buffer. They were then blocked and stained with antibodies and viewed.
Semi-quantitative PCR
Total RNA was isolated by using RNase mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. First-strand cDNA synthesis was performed using Verso cDNA synthesis kit (Thermo Fisher Scientific, USA) as per manufacturer's instruction. PCR was done using Emerald Amp Max PCR Master Mix (TakaRa Bio Inc., Shiga, Japan). The following oligonucleotide primers were used for synthesizing respective transcripts
α-fodrin: 5´ TCCCACCAACATCCAGCTTT 3´ (forward),
5´GCCTTGACAGCATCCTCACT3´ (reverse).
GAPDH transcript: 5´GAGTCAACGGATTTGGTCGT3´(forward),
5´GACAAGCTTCCCGTTCTCAG3´ (reverse)
CENPE transcript: 5´ GCCTACAAACCAAAGAAGAA 3´ (forward),
5´TCTTGTAATTTGGTATGGGATTTTT3´ (reverse).
Cyclin B transcript: 5´AGCACCTGGCTAAGAATG 3´ (Forward),
5´ CTTCGATGTGGCATACTTG 3´ (Reverse)
Immunofluorescence
Cells were seeded onto clean sterilized cover slips in 6-well plates or 35 mm dishes. Post 24 h of seeding, the cells were transfected with shRNA. After 96 h of transfection, cells were fixed either with cold methanol or 4% paraformaldehyde. In case of cold methanol fixation, the cells were washed with PBS once and ice cold methanol was added and incubated at −20°C for 10 min. The methanol was then removed and washed with PBS followed by blocking. In case of paraformaldehyde fixation, the cells were washed with PBS followed by incubation with 4% paraformaldehyde in PBS for 14 min at room temperature. The cells were then incubated with PBS containing 0.1% Triton X 100 for 10 min. Subsequently, the cells were washed with PBS followed by blocking. For blocking, the cells were incubated with 10% FBS in PBST (PBS with 0.1% Triton X 100) buffer at room temperature for 30 min at 37°C. They were then incubated overnight with primary antibody diluted in PBST at 4°C. Following primary antibody incubation, the cells were washed thrice in PBS with 0.1% Tween 20 followed by incubation with secondary antibody diluted in PBST at room temperature for 2 hours. After washing with PBS, the cells were mounted with Vectashield containing DAPI. In case of fodrin-depleted cells, metaphase-like cells were selected as those which showed maximum alignment of chromosomes near the metaphase plate.
Transduction of U-251 MG cells
U-251 MG cells were seeded in DMEM with 5% FBS at 2 × 105 cells per well of a 6-well plate devoid of antibiotics. Four hours post seeding, a viral mixture containing pLVX-Tight-Puro-H2B-GFP-α-Tubulin-mcherry, pLVX-Tet-On-Advanced and polybrene (8 μg/mL) was mixed and incubated for 30 min at room temperature to allow complexes of viral particles to form [15]. The mixture was then added to the cells and incubated overnight. Post 12 h, the medium was replaced by fresh DMEM with 10% FBS, antibiotic mix and 350 ng/mL of doxycycline. The cells were then incubated at 37°C for 48–72 h and used for live cell imaging experiments, or passaged and cryopreserved as required.
Live cell imaging
U-251 MG and HeLa stably expressing H2B-GFP and Tubulin-mCherry were seeded in ibidi 2 well μ-Slides (ibidi, Germany) and transfected with control or α-fodrin shRNA and incubated for 72 h. Post 72 h, the cells were incubated with DMEM without phenol red containing 10% FBS and 1% sodium pyruvate, 350 ng/mL of doxycycline and further incubated for 24 h in CO2 incubator. The slide was then imaged using a Nikon eclipse Ti microscope illuminated with LED lamps, fully thermostated and containing a CO2 stage incubator (Okolab, Italy) to maintain the temperature at 37°C and 5% CO2. The images were obtained using 40X or 60X oil immersion objective with LED illumination by SpectraX (Lumencor, USA). Z stacks of 1 μm were used along with channels for GFP and mCherry. The images were taken every 5 min for 16–20 htime period and analyzed using ImageJ. The stacks were deconvoluted using Huygens software (SVI, Netherlands).
Fluorescence intensity measurements
Images were obtained using Zeiss Axio Imager Z1 and Nikon eclipse A1 confocal microscope using 60X or 100X oil immersion objectives. Care was taken while imaging to keep the intensity of light and voltage constant for both control and α-fodrin shRNA treated samples. Maximum intensity profiles were generated for every image before analysis. Images obtained from immunofluorescence experiments were analyzed using ImageJ/Fiji [16]. An ROI (Region Of Interest) of constant size around the CREST staining was drawn and intensity of both CREST as well as protein of interest (BubR1, CENP-E) at the kinetochore position in the ROI was calculated after subtracting the background intensity. Further the ratio of BubR1/CREST or CENP-E/CREST was calculated and analyzed for both control shRNA and α-fodrin shRNA treated cells. On an average, about 75 kinetochores was analyzed using GraphPad Prism and the experiment was repeated thrice. Representative figures were deconvoluted using Huygens software (SVI, Netherlands). For determining detyrosinated α-tubulin levels on the spindle, a rectangular ROI was drawn covering the centrosome along with the proximal end of the spindle. The intensity of detyrosinated α-tubulin was normalized against that of α-tubulin in the demarcated ROIs in both control and α-fodrin deficient cells. On an average around 30, such ROI was measured. Experiment was repeated thrice.
Mitotic progression
U-251 MG and HeLa cells expressing H2B-GFP and Tubulin-mcherry were transfected with control or α-fodrin shRNA. After 96 h, they were subjected to live cell imaging as described above. Images of mitotic cells were collected at a time interval of 5 min. The images were stacked and maximum intensity profiles were generated and processed using ImageJ. The number of frames required for a cell undergoing nuclear envelope breakdown (NEB) to onset of anaphase as evident from H2B-GFP staining was calculated manually. The number of frames was multiplied by 5 to get the time in minutes for NEB to anaphase onset.
Analysis of data
The blots were quantified by densitometric analysis using Bio-Rad’s Quantity One software and they were normalized with actin or GAPDH wherever necessary. For imaging, events were counted manually wherever required and for statistical analysis and graph preparations, GraphPad Prism was used. Unpaired Student’s t-test was used for finding the statistical significance and p ≤ 0.05 were considered as statistically significant.
Results
α-Fodrin repression causes mitotic spindle defects
Apart from its structural roles, several studies have implicated spectrin and fodrin in cell adhesion, cell signaling and cell proliferation [1,11]. Fodrin is known to bind to microtubules [14] but the effect of α-fodrin on microtubule organization and function in mitotic cells remains unexplored. Towards this objective, we first tested whether fodrin co-localizes with microtubules in mitotic U251 MG cells. As shown in Figure S1, fodrin co-localizes with microtubules in mitosis. Next, we depleted endogenous α-fodrin in U251 MG cells using both single shRNA or a mixture of 3 different shRNAs. Both single and mixed shRNA treatment efficiently depleted α-fodrin (Figure 1(a)). We also observed a reduced mitotic index in α-fodrin-depleted cells when compared to control (Figure 1(b)). Moreover, mitotic cells exhibited significant spindle abnormalities, such as multipolar spindles, and chromosome misalignment (Figure 1(c,d)). Mitotic phenotypes were categorized into normal, misaligned or multipolar. Whereas control shRNA-treated cells showed reduced levels of misaligned or multipolar spindles, α-fodrin shRNA-depleted cells most often exhibited chromosome misalignment, and also significant increase of spindle multipolarity. α-Fodrin is known to interact with both tubulin and actin, and we found α-fodrin to affect MT organization. Therefore, we examined the global levels of α- and γ-tubulin, as well as of actin upon fodrin depletion. We found no significant changes in the expression levels of these proteins (Figure 1(e)). All the experiments were replicated in mitotic IMR-32 cells, which gave similar results (Figure S2A-D). A rescue experiment for the shRNA could not be successfully done as we were unable to transduce the big clone of α-fodrin (12.05 KB) or α-fodrin mutant in glioblastoma cell line U251 MG or neuroblastoma cell line IMR-32. Nevertheless, our data suggest that α-fodrin is involved in the organization of MT in mitosis.
Figure 1.
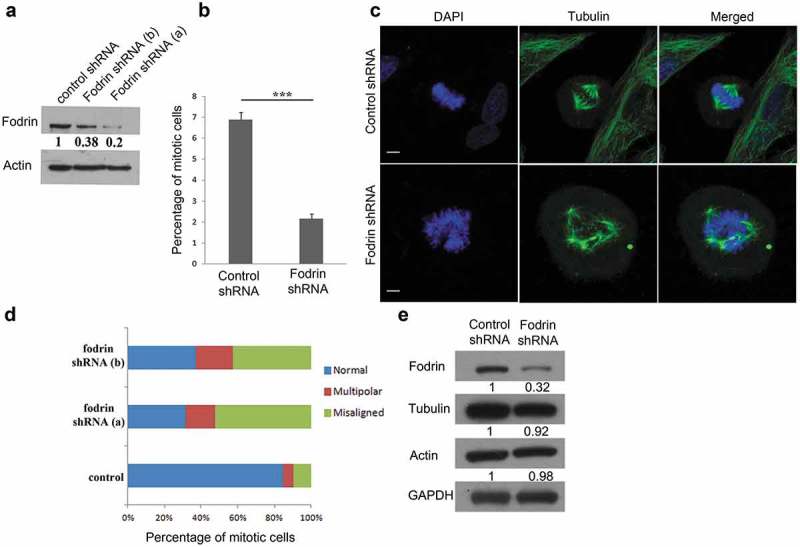
Depletion of α-fodrin leads to abnormal mitosis. (a) Blot showing downregulation of α-fodrin in U-251 MG cell line using single shRNA targeted against fodrin (shRNA a) and mixture of 3 shRNA (shRNA b) with actin as loading control. (b) U-251 MG cells were subjected to α-fodrin downregulation using shRNA for 96 h and cells were fixed using ice cold methanol. They were then stained with α-tubulin antibody and nuclei were stained with DAPI. Quantitative analysis of the percentage of mitotic cells in control and fodrin shRNA-treated cells using shRNA a. (c) Mitotic abnormalities observed in case of fodrin depleted cells using shRNA b. The experiment was repeated thrice. (d) Quantitative analysis of the percentage of normal, misaligned and multipolar cells in control and fodrin shRNA-treated cells. N = 125 and the experiment was repeated thrice. (e) Western blot showing no change in tubulin and actin levels upon fodrin depletion by shRNA with a GAPDH as loading control. For statistical analysis, Student's t-test was carried out and * represents p < 0.05 and *** represents p < 0.001. Scale bar represents 5 μm.
Depletion of α-fodrin delays mitotic progression
To further characterize the spindle defects observed upon α-fodrin depletion, we followed mitotic progression by live cell imaging of U251 MG cells expressing H2B-GFP/Tubulin-mCherry after treatment with either control or α-fodrin shRNAs. Since we got maximum downregulation with single shRNA (shRNA a), further downregulation experiments were performed using the same. Consistent with fixed cell analysis, α-fodrin downregulated cells exhibited increased mitotic defects when compared to control cells (Figure 2(a)). While most of the control cells (A1,A2 and M1) show chromosome alignment within 30 min after mitotic entry, α-fodrin-depleted cells show either a consistent delay in congression and mitotic exit (A3 and M2) or fail to align their chromosomes and do not exit mitosis even after 3.5 h (A4 and M3) (Figure 2(b)). Statistical analysis showed that for the cells that managed to exit mitosis, the time taken from nuclear envelope breakdown (NEB) to anaphase onset was significantly longer upon α-fodrin depletion (50.07 ± 2.8 min), as compared to control cells (30.67 ± 0.9 min) (Figure 2(c)). However, we also found 18% of α-fodrin depleted cells not to progress into anaphase within the time frame of the experiment (3.5 h) as opposed to only 1% in control cells (Figure 2(d)). Similar experiments were also carried out using HeLa H2B-GFP/Tubulin-mCherry cells where α-fodrin downregulation to 80% of control levels was achieved (Figure S3A). These cells also exhibited chromosome congression defects, delayed progression through mitosis (40%) and mitotic arrest for >3.5 h (26%) (Figure S3B-D). These results suggest that α-fodrin depletion severely impairs mitotic progression.
Figure 2.
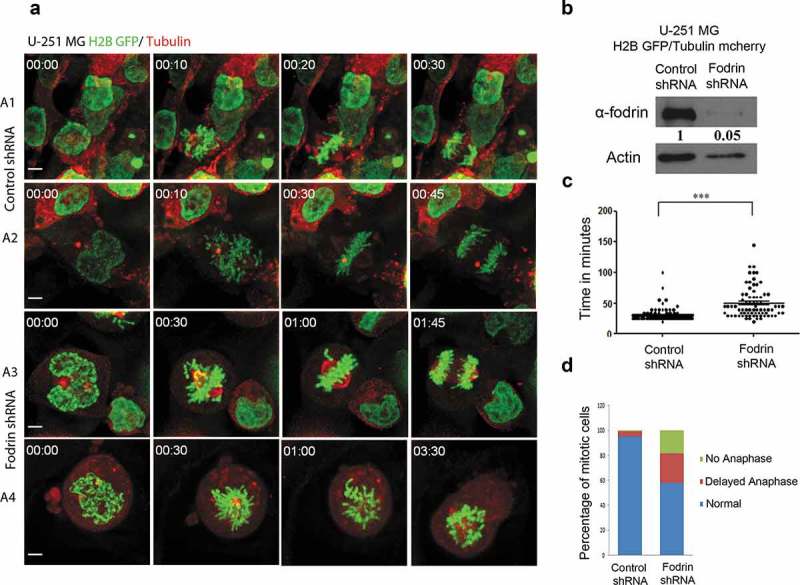
α-fodrin depletion causes delayed mitotic progression. U-251 MG H2B-GFP Tubulin-mcherry cells were transfected with either control or fodrin shRNA and post 96 h, live cell imaging was performed and time taken for progression from nuclear envelope breakdown (NEB) to onset of anaphase was calculated. (a) Different time frames of live cell imaging for control and fodrin silenced cells. (b) Blot showing more than 90% downregulation of fodrin in U-251 MG H2B GFP/Tubulin mCherry cells using shRNA a. (c) Graph depicting the time taken in minutes for NEB to onset of anaphase. N = 119 for control and N = 76 for fodrin-depleted cells. Experiment repeated thrice. (d) Bar graph depicting the percentage of cells undergoing normal, delayed and arrested mitotic progression. Sixty minutes and more time taken for nuclear envelope breakdown (NEB) to onset of anaphase was arbitrarily taken as delayed. Cells that stayed more than 3 h or more in metaphase were considered as arrested. Student's t-test was performed and *** stands for p < 0.001. Scale bar represents 5 μm.
α-Fodrin depletion results in activation of the spindle assembly checkpoint
Since depletion of α-fodrin compromises chromosome alignment and spindle assembly, with a significant delay in mitotic progression, we investigated the status of the Spindle Assembly Checkpoint (SAC). The SAC is a highly sensitive mechanism that prevents mitotic exit until all chromosomes congress to the metaphase plate and show bipolar microtubule attachment [17,18]. To determine whether the mitotic delay upon α-fodrin depletion is due to SAC activity, cells were treated with Reversine, an inhibitor of Mps1 kinase [19]. Mps1 is an upstream orchestrator of SAC signaling and its activity is high at unattached kinetochores to instate SAC response and the activity dramatically decreases following microtubule attachment [20–22]. Mps1 inhibition should allow cells to override the checkpoint and exit mitosis [23]. We found that cells depleted of α-fodrin incubated with reversine no longer experienced delay or arrest in mitosis (Figure 3(a,b)). Cells depleted of α-fodrin showing either major failure in chromosome alignment or misalignment of few chromosomes, bypassed the SAC and entered anaphase upon addition of reversine. To further confirm SAC activation, we examined BubR1 immunostaining at kinetochores of α-fodrin-depleted mitotic cells. BubR1 is a SAC protein that strongly accumulates at kinetochores when SAC is active. It plays a vital role in the inhibition of the anaphase-promoting complex/cyclosome (APC/C), delaying the onset of anaphase and ensuring proper chromosome segregation until all chromosomes are properly bi-oriented on the mitotic spindle. Its level decreases when the chromosomes are properly attached to the kinetochores [24,25]. As expected, α-fodrin-depleted prometaphase cells exhibited similar levels of BubR1 staining at unattached kinetochores in comparison to control. However, in contrast to metaphase control cells, α-fodrin-depleted defective metaphase cells exhibited high levels of BubR1, suggesting that depletion of α-fodrin prevents the formation of proper end-on kinetochore attachments and SAC inactivation (Figure 3(c,d)).
Figure 3.
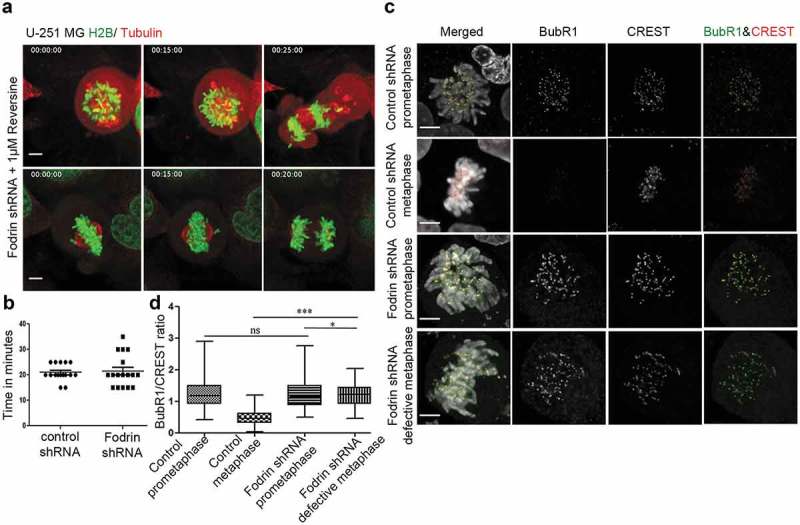
Activation of spindle assembly checkpoint upon α-fodrin depletion. U-251MG H2B-GFP/Tubulin-mcherry cells were subjected to fodrin downregulation using fodrin shRNA for 96 h as in Figure 2 and they were imaged in 60X objective for 10 h using Nikon Ti epifluorescence microscope with stage saturated with 5% CO2 and maintained at 37°C. When the cells showed abnormalities, they were treated with 1 μM Reversine and imaged for further 6 h. (a) Mitotic cells with abnormalities exit mitosis upon reversine treatment. (b) Graph depicting duration for NEB to anaphase onset in control and fodrin shRNA-treated cells after reversine treatment. N = 16 for control and N = 17 for fodrin-depleted cells. (c) Immunofluorescence imaging of control and fodrin depleted cells. Cells were stained for BubR1 and CREST while DAPI was used for staining of the nucleus. They were then imaged in 60X objective using Zeiss epifluorescence microscope. (d) Box and whiskers plot depicting the increased level of BubR1 localization at the kinetochores of defective metaphases in fodrin downregulated U-251 MG cells. Fifteen kinetochores per cell were taken. A small (however significant) decrease was seen from fodrin-depleted prometaphase cells to defective metaphase cells. Total number of kinetochores N = 200 for downregulated fodrin metaphase condition, N = 250 for other condition. All experiments were repeated thrice. *** stands for p ≤ 0.001 and * stands for p ≤ 0.05. Scale bar represents 5 μm.
To further examine the defects in kinetochore-MT attachments, we performed a calcium treatment assay in permeabilization buffer, which selectively depolymerizes most microtubules, except those that are stably attached to kinetochores. We found that after calcium treatment, control cells exhibited aligned chromosomes with a significant proportion of kinetochore fibers (Figure 4(a)), whereas after α-fodrin depletion, chromosomes were rarely aligned with decreased number of kinetochore fibers (Figure 4(a,b)). We quantified the ratio of unbound kinetochores to the total kinetochore number, and found this ratio to be significantly increased following α-fodrin depletion, in agreement with the increased levels of BubR1 observed in the defective metaphase cells (Figure 3(d)).
Figure 4.
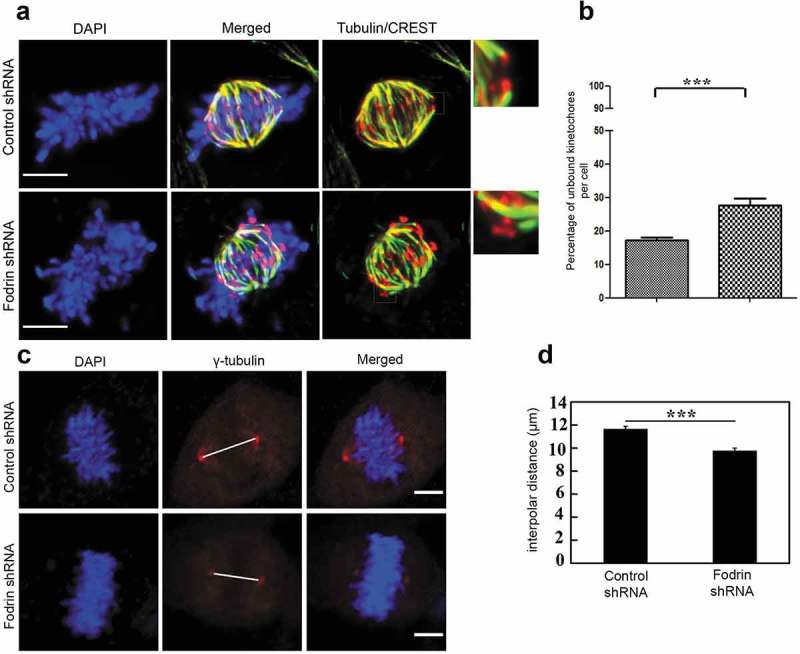
Depletion of α-fodrin hampers microtubule kinetochore attachment. (a) Immunostaining for tubulin and CREST using 100X objective of Zeiss epifluorescence microscope to determine the microtubule bound kinetochores. The smaller panels in the right side show the zoomed in images of the square boxes of microtubule bound kinetochore in case of control and microtubule unbound kinetochore in case of fodrin-depleted cells. (b) Graph depicting increased percentage of microtubule unbound kinetochore in fodrin downregulated U-251 MG cells. N = 14 for control, N = 15 for fodrin-depleted cells. (c) Immunofluorescence imaging of γ-tubulin in control cells and fodrin shRNA-treated cells to determine interpolar distance. The length of the line between the two centrosomes was considered as the spindle length. (d) Interpolar distance was calculated between the two centrosomes stained by γ-tubulin and measured using ImageJ. N = 50. shRNA treatment conditions are same as in Figure 2. All experiments were repeated thrice. *** stands for p ≤ 0.001. Scale bar represents 5 μm.
For proper bipolar spindle assembly, the mechanical force generated by kinetochore-MT attachments is important. Different mitotic proteins give rise to different effects on spindle length. Such cases have been reported in the literature, where the reduced expressions of certain microtubule-binding proteins such as EB1, Eg5, Mast and Msps have shown to cause shortened spindles [26,27]. The lack of tension at unattached kinetochores should induce spindle length reduction in α-fodrin-depleted cells. Indeed, α-fodrin-depleted metaphase-like cells exhibited shortened spindles as determined by interpolar distance (Figure 4(c,d)). Altogether, our data show that α-fodrin repression compromises kinetochore-MT attachment stability leading to prolonged SAC activation and mitotic delay.
α-Fodrin depletion inhibits CENP-E localization at kinetochores
The role of motor proteins in spindle formation and chromosome alignment during mitosis is well established. Amongst the motor proteins involved, CENP-E is the kinesin motor that primarily contributes to the movement of chromosomes towards the metaphase plate [28–31]. Previous studies have shown that loss of CENP-E particularly delays the congression of chromosomes that are proximally located to the spindle poles [32,33]. Furthermore, a role for CENP-E in maintaining stable microtubule-kinetochore interactions has been documented [34,35]. We, therefore, asked whether CENP-E could account for the kinetochore-microtubule attachment defects in α-fodrin-depleted cells. Immunostaining of CENP-E in control and α-fodrin-depleted cells revealed that whereas CENP-E accumulated strongly in kinetochores of prometaphase control cells, CENP-E levels were significantly reduced in α-fodrin-depleted prometaphase cells. qPCR analysis of CENP-E RNA levels showed no significant alteration between control and α-fodrin-depleted cells (Figure 5(c)) suggesting that CENP-E is either delocalized or reduced at the protein level. Thus, our data suggest that failure to accumulate CENP-E at kinetochores in prometaphase likely accounts for the chromosome misalignment phenotype and decreased number of K-fibers in α-fodrin-depleted cells.
Figure 5.
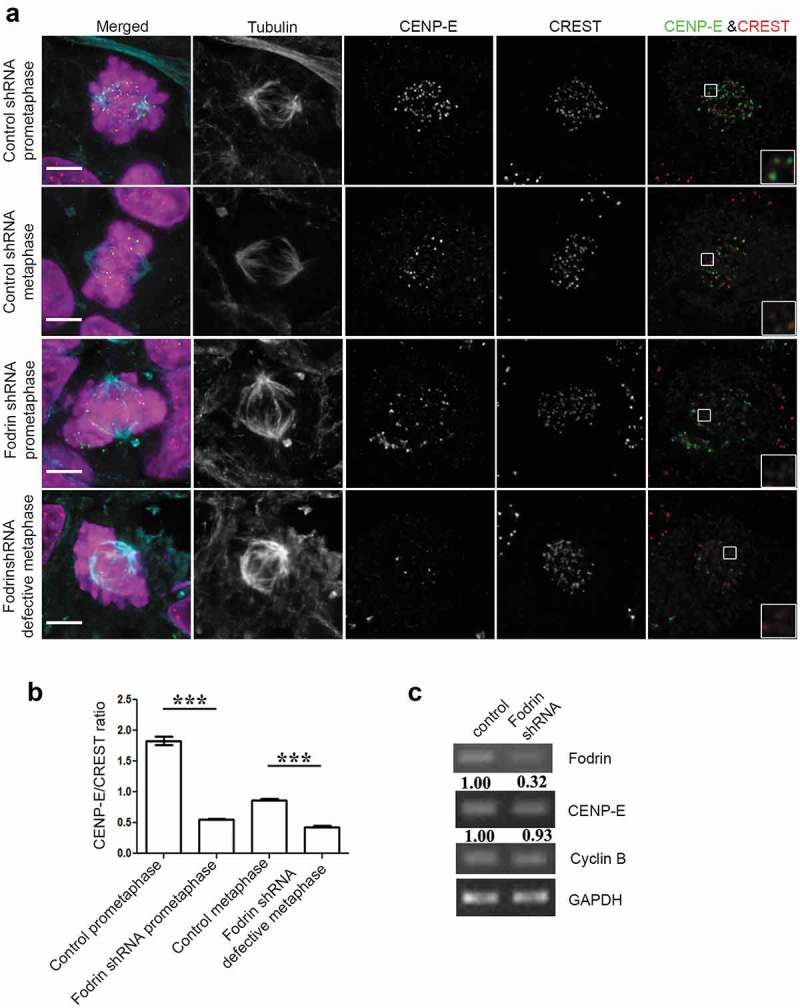
Fodrin depletion reduces localization of CENP-E at the kinetochore region. (a) U-251 MG cells were subjected to α-fodrin downregulation using shRNA for 96 h and cells were fixed using paraformaldehyde. They were then stained for CREST, CENP-E and tubulin. Scale bar represents 5 μm. Insets show zoomed in portion of CENP-E and CREST staining which clearly suggest a decrease in CENP-E in fodrin-depleted condition. (b) Bar graph representing the CENP-E to CREST ratio. N = 125. (c) Total RNA was prepared from α-fodrin-depleted U-251 MG cells and subjected to qPCR using primers against α-fodrin, GAPDH, Cyclin B and CENP-E. shRNA treatment condition was same as in Figure 2. All experiments were repeated thrice. *** stands for p ≤ 0.001.
α-Fodrin repression compromises tubulin detyrosination
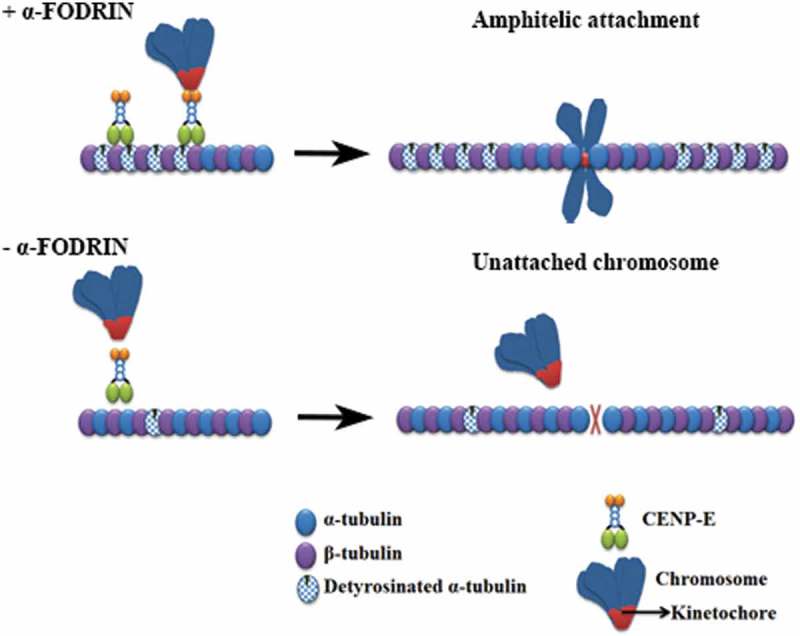
Despite the significant decrease in CENP-E localization at the kinetochores in α-fodrin-depleted cells, a direct interaction between α-fodrin and CENP-E could not be detected (data not shown), thus suggesting that α-fodrin affects CENP-E localization by some other mechanism. Tubulin post-translational modifications have been reported to modulate the activity of motor proteins. Kinesins have been reported to be regulated by the acetylation and detyrosination status of tubulin [36,37]. CENP-E requires detyrosinated α-tubulin in the spindle fibers for its movement [38]. We thus tested the effect of α-fodrin depletion on detyrosinated α-tubulin. Immunofluorescence analysis showed decreased levels of detyrosinated α-tubulin on spindle fibers of α-fodrin-depleted cells (Figure 6(a)). Quantitative analysis of the ratio of detyrosinated α-tubulin to tubulin showed significant reduction in α-fodrin-depleted cells when compared to control cells (Figure 6(b)). Total protein levels also showed reduced detyrosinated α-tubulin levels in α-fodrin-depleted cells (Figure 6(c)). Thus, α-fodrin repression compromises tubulin detyrosination which may be the cause for defective CENP-E localization and chromosome congression delay.
Figure 6.
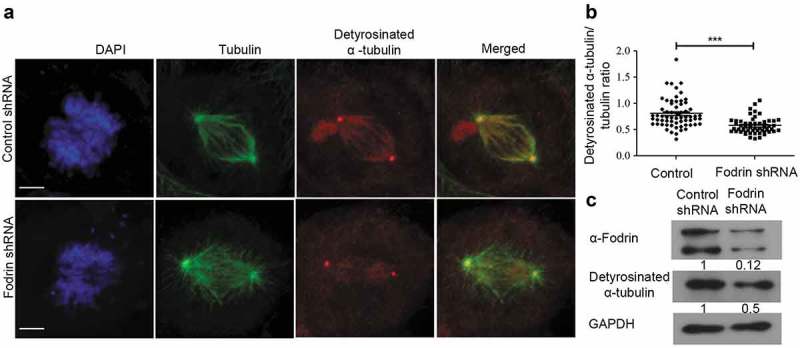
Effect of α-fodrin silencing on post-translationally modified tubulin. (a) U-251 MG cells were subjected to α-fodrin downregulation using shRNA same as in Figure 2 for 96 h. Cells were fixed using methanol and stained for detyrosinated α-tubulin and α-tubulin. Scale bar represents 5 μm. (b) Graph representing the detyrosinated α-tubulin to α-tubulin ratio. N = 30. (c) Western blot showing fodrin downregulation in absence of calpain inhibitor and levels of detyrosinated α-tubulin with GAPDH as loading control. The lower band in fodrin blot detects cleaved fodrin as is found in most of the fodrin treatments. All experiments were repeated thrice. *** stands for p ≤ 0.001.
Discussion
Fodrin is a cytoskeleton protein that is known to provide structural framework to cells [1,39]. Recent work has also revealed the role of fodrin in cellular and organ growth. Homozygous knockout of α-fodrin in mice is embryonically lethal causing major impairments in heart and brain development [40]. Beyond organ development, α-fodrin has been documented to be involved in cell cycle progression and DNA repair mechanisms. It interacts with many cytoskeleton components, including microtubules involved in organelle and macromolecular transport, migration and cell division [30,41–43]. Moreover, α-fodrin causes bundling of purified microtubules [14]. However, the functional regulation of microtubules by α-fodrin, and in particular mitotic spindle microtubules, has remained unknown. Earlier studies had shown that fodrin depletion caused cells to accumulate in G1 [11] which is accounted by increased levels of the CDK inhibitor p21, and a reduction of cells in the G2/M and S phase. In this study, we studied the effect of α-fodrin depletion on mitotic progression and organization. As per Metral et al., we also found a decrease in the mitotic population after fodrin depletion. As we focussed on this limited population of mitotic cells, we found that α-fodrin repression significantly interfered with chromosome congression. Furthermore, we observed a significant increase in the duration of prometaphase with a significant number of cell failing chromosome congression altogether. Even when all chromosomes were apparently aligned, an elevated percentage of α-fodrin-depleted cells failed to proceed to anaphase due to a persistent SAC activation with increased levels of BubR1 at kinetochores. In agreement, calcium treated cells revealed that in the absence of α-fodrin, the stability of kinetochore-microtubule attachment was severely compromised. This was also reflected in the reduced spindle length of α-fodrin-depleted mitotic cells. CENP-E, one of the major motor proteins involved in transporting the chromosomes from the spindle poles to the metaphase plate [29,30,44], was found to be significantly reduced at kinetochores of α-fodrin-depleted cells. Interestingly, in agreement with the putative role of α-fodrin in regulating CENP-E activity in mitosis, previous comparative proteome analysis of chromosome bound proteins have identified both CENP-E and α-fodrin among a group of 66 proteins [45]. Persistent SAC activation as shown in Figure 3 indeed happens in the lower number of mitotic cells that are observed upon fodrin depletion. The reason for lower mitotic index is not clear at this moment. Our future studies would detect whether these cells undergo apoptosis.
CENP-E preferentially moves on detyrosinated microtubules of the spindle to move chromosomes towards the metaphase [38]. Here, we show that depletion of fodrin makes chromosomes unable to congress at the metaphase plate probably due to a decrease in both CENP-E and overall detyrosinated spindle tubulin level. The enzyme tubulin carboxypeptidase (TCP) removes tyrosine from microtubules and a depletion of TCP would have less detyrosinated tubulin. Indeed, α-fodrin-depleted cells are similar to TCP depleted cells. Thus, we speculate that a reduction of detyrosinated tubulin upon fodrin depletion causes a reduction in CENP-E amount.
It is known that detyrosinated tubulin makes microtubules less dynamic [46] and with this form of post-translationally modified tubulin, the microtubules are more stable even though the cause is not known [47]. Since α-fodrin downregulation reduces detyrosinated tubulin, it would be interesting to see whether the microtubules in fodrin-depleted cells are more dynamic and whether the microtubules formed after fodrin depletion are less stable. Since we saw this effect in mitotic cells, it is also possible that fodrin depletion effects posttranslational modification of microtubules in interphase cells by regulating the detyrosination/tyrosination cycle through its action on tubulin carboxypeptidase or tubulin tyrosine ligase.
The role of fodrin in microtubule organization is further supported by our previous observations where we showed that it is a constituent of brain γ-tubulin ring complex [12,13]. Moreover, we have also shown that fodrin interacts directly with γ-tubulin in brain-derived cell lines and that the interaction of fodrin with gamma-tubulin is responsible for its inhibitory effect on γ-tubulin-mediated microtubule nucleation [48]. However, so far we have been unable to find co-localization of fodrin with γ-tubulin in cell lines other than those derived from brain. Nevertheless, the mitotic defects that we describe here were found in HeLa cells, as well as in brain-derived cell lines. Therefore, it is reasonable to assume that at least during mitosis, α-fodrin effect is independent of cell and tissue identity.
Thus, we have presented a so far undisclosed role of α-fodrin in the regulation of chromosome alignment and metaphase completion, raising important questions for future research on the mechanisms behind the cross-talk of different cytoskeleton components and its clinical significance in cancer.
Funding Statement
RKN, JSS and DD got fellowships from University Grants Commission, India. RKN was also funded by European Molecular Biology Organization short term fellowship (No. 7103). [IF/00916/2014]; Rajiv Gandhi Centre for Biotechnology, Government of India; North Regional Operational Program [NORTE-01-0145-FEDER-000029]; Fundação para a Ciência e a Tecnologia (PT) [PTDC/BIA-CEL/31120/2017]; North Regional Operational Program [NORTE-01-0145-FEDER-000029]; Fundação para a Ciência e Tecnologia grant [PTDC/ BEX- BCM/1921/ 2014]; DST-SERB [EMR/2015/001676]; Fundação para a Ciência e a Tecnologia (PT) [PTDC/BEX-BCM/2090/2014].
Acknowledgments
We acknowledge the help provided by Dr. Paula Sampaio for live cell imaging and Ms. Bindu Ashokan for fixed cell imaging.
Disclosure statement
No potential conflict of interest was reported by the authors.
Summary statement
The cortical cytoskeleton protein α-fodrin plays a vital role in mediating proper chromosome alignment and timely mitotic progression by affecting the organization of spindle microtubule arrays.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Zhang R, Zhang C, Zhao Q, et al. Spectrin: structure, function and disease. Sci China Life Sci. 2013;56:1076–1085. [DOI] [PubMed] [Google Scholar]
- [2].Machnicka B, Grochowalska R, Bogusławska DM, et al. Spectrin-based skeleton as an actor in cell signaling. Cell Mol Life Sci. 2012;69:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ipsaro JJ, Mondragón A.. Structural basis for spectrin recognition by ankyrin. Blood. 2010;115:4093–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mutha S, Langston A, Bonifas JM, et al. Biochemical identification of alpha-fodrin and protein 4.1 in human keratinocytes. J Invest Dermatol. 1991;97:383–388. [DOI] [PubMed] [Google Scholar]
- [5].Kim SS, Shetty K, Katuri V, et al. TGF-beta signaling pathway inactivation and cell cycle deregulation in the development of gastric cancer: role of the beta-spectrin, ELF. Biochem Biophys Res Commun. 2006;344:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dubielecka PM, Grzybek M, Kolondra A, et al. Aggregation of spectrin and PKCtheta is an early hallmark of fludarabine/mitoxantrone/dexamethasone-induced apoptosis in Jurkat T and HL60 cells. Mol Cell Biochem. 2010;339:63–77. [DOI] [PubMed] [Google Scholar]
- [7].Tang Y, Katuri V, Dillner A, et al. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–577. [DOI] [PubMed] [Google Scholar]
- [8].Sridharan D. Nonerythroid alphaII spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links. J Cell Sci. 2003;116:823–835. [DOI] [PubMed] [Google Scholar]
- [9].McMahon LW, Sangerman J, Goodman SR, et al. Human α Spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links †. Biochemistry. 2001;40:7025–7034. [DOI] [PubMed] [Google Scholar]
- [10].McMahon LW, Zhang P, Sridharan DM, et al. Knockdown of alphaII spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem Biophys Res Commun. 2009;381:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Metral S, Machnicka B, Bigot S, et al. AlphaII-spectrin is critical for cell adhesion and cell cycle. J Biol Chem. 2009;284:2409–2418. [DOI] [PubMed] [Google Scholar]
- [12].Thomas NE, Shashikala S, Sengupta S. Cytoplasmic gamma-tubulin complex from brain contains nonerythroid spectrin. J Cell Biochem. 2010;110:1334–1341. [DOI] [PubMed] [Google Scholar]
- [13].Shashikala S, Kumar R, Thomas NE, et al. Fodrin in centrosomes: implication of a role of fodrin in the transport of gamma-tubulin complex in brain. PLoS One. 2013;8:e76613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ishikawa M, Murofushi H, Sakai H. Bundling of microtubules in vitro by fodrin. J Biochem. 1983;94:1209–1217. [DOI] [PubMed] [Google Scholar]
- [15].Macedo JC, Vaz S, Bakker B, et al. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat Commun. 2018;9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Musacchio A. The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol. 2015;25:R1002–R1018. [DOI] [PubMed] [Google Scholar]
- [18].Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol. 2012;22:R966–R980. [DOI] [PubMed] [Google Scholar]
- [19].Santaguida S, Tighe A, D’Alise AM, et al. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol. 2010;190:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ji Z, Gao H, Yu H. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science. 2015;348:1260–1264. [DOI] [PubMed] [Google Scholar]
- [21].Moura M, Osswald M, Leça N, et al. Protein phosphatase 1 inactivates Mps1 to ensure efficient spindle assembly checkpoint silencing. Elife. 2017;6:e25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hiruma Y, Sacristan C, Pachis ST, et al. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science. 2015;348:1264–1267. [DOI] [PubMed] [Google Scholar]
- [23].Jelluma N, Dansen TB, Sliedrecht T, et al. Release of Mps1 from kinetochores is crucial for timely anaphase onset. J Cell Biol. 2010;191:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bolanos-Garcia VM, Blundell TL. BUB1 and BUBR1: Multifaceted kinases of the cell cycle. Trends Biochem Sci. 2011;36:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen RH. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J Cell Biol. 2002;158:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goshima G, Scholey JM. Control of mitotic spindle length. Annu Rev Cell Dev Biol. 2010;21–57. [DOI] [PubMed] [Google Scholar]
- [27].Goshima G, Wollman R, Stuurman N, et al. Length control of the metaphase spindle. Curr Biol. 2005;15:1979–1988. [DOI] [PubMed] [Google Scholar]
- [28].Yen TJ, Li G, Schaar BT, et al. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992;359:536–539. [DOI] [PubMed] [Google Scholar]
- [29].Schaar BT, Chan GKT, Maddox P, et al. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yao X, Anderson KL, Cleveland DW. The microtubule-dependent motor centromere-associated protein E (CENP- E) is an integral component of kinetochore corona fibers that link centromeres to spindle microtubules. J Cell Biol. 1997;139:435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wen B, Lampe JN, Roberts AG, et al. Knockdown of alphaII spectrin in normal human cells by siRNA leads to chromosomal instability and decreased DNA interstrand cross-link repair. Biochem Biophys Res Commun. 2007;454:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McEwen BF, Chan GK, Zubrowski B, et al. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell. 2001;12:2776–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tanudji M, Shoemaker J, L’Italien L, et al. Gene Silencing of CENP-E by Small Interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay. Mol Biol Cell. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu D, Ding X, Du J, et al. Human NUF2 interacts with centromere-associated protein E and is essential for a stable spindle microtubule-kinetochore attachment. J Biol Chem. 2007;282:21415–21424. [DOI] [PubMed] [Google Scholar]
- [35].Vitre B, Gudimchuk N, Borda R, et al. Kinetochore-microtubule attachment throughout mitosis potentiated by the elongated stalk of the kinetochore kinesin CENP-E. Mol Biol Cell. 2014;25:2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wilson PJ, Forer A. Acetylated α-tubulin in spermatogenic cells of the crane fly Nephrotoma suturalis: Kinetochore microtubules are selectively acetylated. Cytoskeleton. 1989;14:237–250. [Google Scholar]
- [37].Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Barisic M, Silva E Sousa R, Tripathy SK, et al. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science. 2015;348:799–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Burns NR, Ohanian V, Gratzer WB. Properties of brain spectrin (fodrin). FEBS Lett. 1983;153:165–168. [DOI] [PubMed] [Google Scholar]
- [40].Stankewich MC, Cianci CD, Stabach PR, et al. Cell organization, growth, and neural and cardiac development require II-spectrin. J Cell Sci. 2011;124:3956–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vasiliev JM, Samoylov VI. Regulatory functions of microtubules. Biochem Biokhimii͡a. 2013;78:37–40. [DOI] [PubMed] [Google Scholar]
- [42].Etienne-Manneville S. Microtubules in Cell Migration. Annu Rev Cell Dev Biol. 2013;29:471–499. [DOI] [PubMed] [Google Scholar]
- [43].Wittmann T, Hyman A, Desai A. The spindle: A dynamic assembly of microtubules and motors. Nat Cell Biol. 2001;3:E28–E34. [DOI] [PubMed] [Google Scholar]
- [44].Lombillo VA, Nislow C, Yen TJ, et al. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol. 1995;128:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Morrison C, Henzing AJ, Jensen ON, et al. Proteomic analysis of human metaphase chromosomes reveals topoisomerase II alpha as an Aurora B substrate. Nucleic Acids Res. 2002;30:5318–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kreis TE. Microtubules containing detyrosinated tubulin are less dynamic. Embo J. 1987;6:2597–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Peris L, Wagenbach M, Lafanechère L, et al. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sreeja JS, Nellika RK, John R, et al. Binding of alpha Fodrin to gamma Tubulin accounts for its role in the inhibition of microtubule nucleation. Febs Letters. 2019;593:1154–1165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


