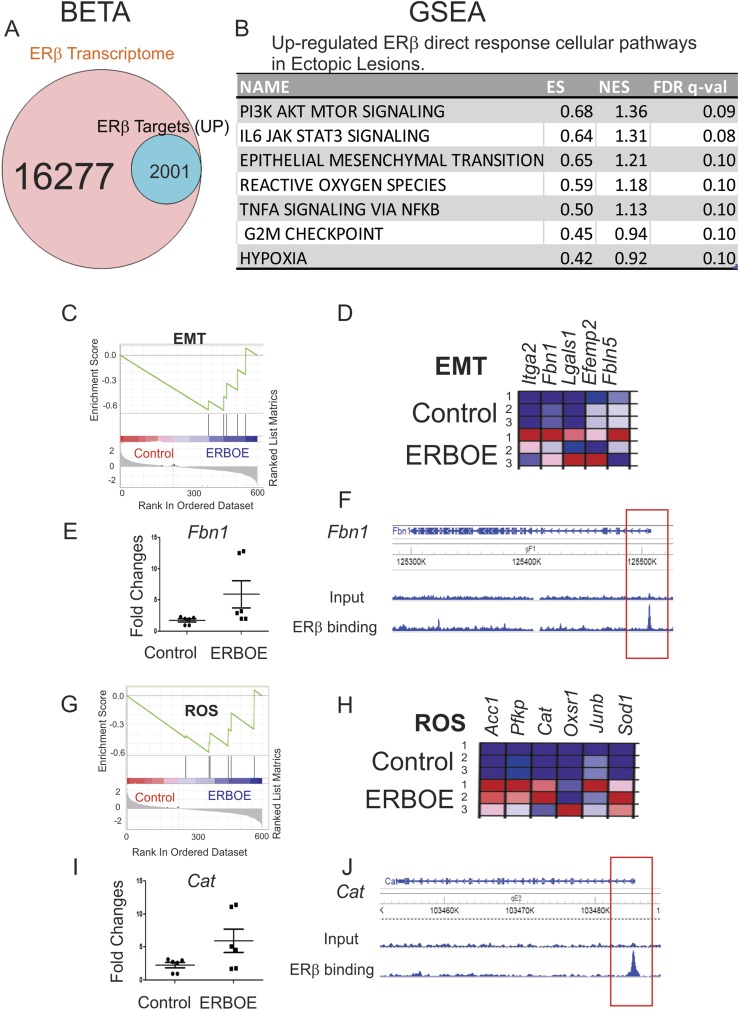
Figure 5.
Cellular pathways directly upregulated to ERβ in ectopic lesions. (A) BETA with ERβ transcriptome and ERβ cistrome in ectopic lesions showed that 12.3% (2001 of 16,277) of genes were upregulated by ERβ and statistically enriched near the ERβ binding sites (P < 0.05) in ectopic lesions. (B) GSEA analysis with 2001 transcripts revealed specific cellular pathways upregulated by direct ERβ target genes in ERβ-overexpressing ectopic lesions. (C) GSEA analysis revealed that ERβ overexpression elevated genes signature associated with the EMT signaling in ectopic lesions. (D) Relative expressions of the EMT gene set in control vs ERβ-overexpressing ectopic lesions (N = 3) are shown in order of their signal/noise ratio rank. (E) The expression of Fbn1 determined by RT-qPCR was elevated in ERBOE ectopic lesions compared with ectopic control lesions. The fold changes are shown relative to Fbn1 level in ectopic control lesions. (F) WashU Epigenome Browser image of ChIP-seq peak data revealed that ERβ bound to the promoter region of Fbn1 gene loci in ectopic lesions compared with the input signal. (G) GSEA analysis revealed that ERβ stimulated the gene signature associated with ROS in ectopic lesions. (H) Relative expressions of the ROS gene set in control vs ERβ overexpressing ectopic lesions (N = 3) are shown in order of their signal/noise ratio rank. (I) Expression of Cat was elevated in ERBOE ectopic lesions compared with ectopic control lesions. The fold changes are shown relative to Cat level in ectopic control lesions. (J) WashU Epigenome Browser image of ChIP-seq peak data revealed that ERβ bound to the promoter region of Cat gene loci ectopic lesions compared with the input signal. ES, enrichment score; FDR, false discovery rate; NES, normalized enrichment score; ROS, reactive oxygen species.