Introduction
Sexual dysfunction is defined as any persistent problem that arises during the sexual response cycle and contributes negatively to sexual performance. Sexual dysfunction occurs in both sexes and can be grouped into four categories (desire, arousal, orgasm and pain problems) [1]. In males, sexual dysfunction may include the inability to achieve or maintain an erection suitable for intercourse as well as retarded, early or premature ejaculation. In comparison, females may experience symptoms such as the inability to achieve orgasm and vaginal dryness. Cardiovascular diseases (CVD) and associated risk factors such as atherosclerosis, hypertension, diabetes and obesity are closely linked with male and female sexual dysfunction (FSD) [2]. Vascular health plays an important role in sexual function; for instance, inflammation-induced endothelial injury is a major contributor for sexual dysfunction in CVD (Figure 1). Recent papers have reviewed the role of inflammation on sexual dysfunctions [3,4], however, the pivotal role of innate immune system, specially the Toll-like receptors (TLR), in initiating the vascular and non-vascular impairments related to sexual dysfunction was not addressed. Moreover, the TLR activation by damage-associated molecular patterns (DAMPs) and oxidized lipoproteins, and the regulation of TLR by hormones in male and female sexual organs were also not considered. Therefore, the aim of this review is to better understand the correlation between sexual dysfunction and inflammation in vascular disease, specifically on vascular inflammatory response elicited by the innate immune system and its relationship with sexual dysfunction.
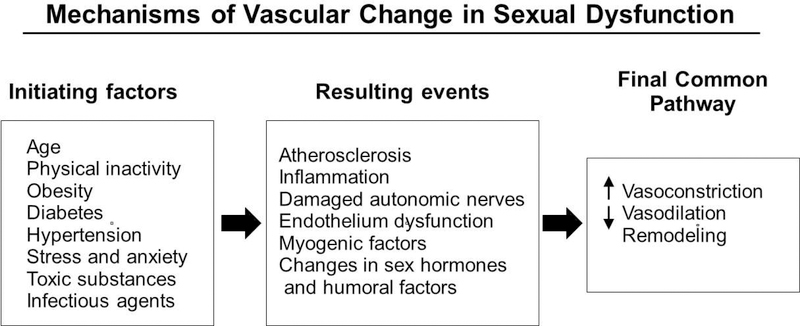
Figure 1.
Mechanisms of vascular change in sexual dysfunction. Initiating factors that contribute to sexual dysfunction in both sexes include: age, physical inactivity, obesity, diabetes, hypertension, stress and anxiety, toxins and infectious agents. These initiating factors may lead to resulting events that end in a final common pathway of vascular dysfunction.
Phenotypical differences between male and female sexual organs occur around the 9th week of gestation. The clitoris is anatomically similar to the penis, even after differentiation, and it was recently described as “the female penis”, composed by two corpora cavernosa and a spongiosal tissue [5]. The male penis is composed of 3 cylinders of erectile sinusoids including two corpus cavernosum (CC), the corpus spongiosum, glans and prepuce, surrounded by a bi-layered fibro-skeleton structure (the tunica albuginea), which is essential to maintain rigidity during sexual intercourse [6].
Peripherally, the penis is innervated by nerves originating from the spinal cord and ganglia located at S2 to S4, from which the pelvic splanchnic nerves and pudendal nerves originate, respectively [7]. Dorsal nerves arising from the pudendal nerve and cavernosal nerves arising from the autonomic pelvic plexus provide the sympathetic and parasympathetic innervation, respectively, to the penis [7]. In females, clitoral sensation is driven by pudendal, hypogastric and pelvic nerves, while the erectile tissue is innervated by cavernous nerve from the autonomic plexus [8].
Upon sexual stimulation, the central nervous system is activated causing enhanced blood flow to the genital organs, leading to penile erection by the relaxation of the CC in males or engorgement and lubrication of the clitoris and vagina in females [9]. In both males and females, the pudendal artery, which originates from internal iliac artery, supplies blood to sexual tissue; damage or occlusion of which negatively affects sexual function.
In the early 1990’s, nitric oxide (NO) was recognized as the main mediator of penile erection. NO is synthesized and released upon activation of endothelial and neuronal NO synthase (eNOS and nNOS, respectively). Increased NO production targets soluble guanylyl cyclase (sCG) to increase the levels of cyclic GMP (cGMP). Elevated concentrations of cGMP activate protein kinase G and decrease intracellular levels of calcium, which lead to smooth muscle relaxation [10]. The effects of NO on penile erection are reversed by phosphodiesterase type 5 (PDE5), which works by catalyzing the breakdown of cGMP to 5’GMP. Inhibition of the PDE5 enzyme constitutes the current first-line therapy for erectile dysfunction (ED) [11].
In females, the regulation of blood flow to sexual organs is also governed by the NO/cGMP pathway, similar to what is observed in men. Females express high levels of nNOS in nerve bundles that run along the clitoris, which initiate the smooth muscle relaxation and clitoral erection during sexual arousal. The increased shear stress of the blood on the endothelium stimulates eNOS to produce NO in the vasculature, periurethral glans and vaginal epithelium, contributing to maintain the smooth muscle relaxation and clitoral erection [12,13]. The presence of NO pathway-related enzymes in female sexual organs indicates that mechanisms for clitoral engorgement and vaginal lubrication are similar to male erection.
Impact of the innate immune response on sexual dysfunction
ED is correlated with elevated serum levels of inflammatory biomarkers such as the cytokine family, which includes interleukins (IL), interferons (IFN) and tumor necrosis factor-α (TNF-α) [14]. The infusion of TNF-α in wild type mice contributes to endothelial dysfunction and cavernosal smooth muscle (CSM) hypercontractility [15]. TNF-α knockout mice are protected from erectile dysfunction due to increases in CSM nNOS and eNOS protein expression. Further, TNF-α also contributes to angiotensin II-induced CSM impairments [16]. In a rat model of ED through bilateral cavernous nerve injury, Matsui et al reported an increase of pro-inflammatory cytokine gene expression (IL-1β, IL-6 and TNF-α) and macrophage infiltration in the major pelvic ganglion [17]. In type 2 diabetic rats, the overexpression of IL-6 and TNF-α in CC tissue led to ED [18]. Interestingly, PDE5 inhibitors have been shown to decrease inflammatory cell infiltration and cytokine production in several tissues including CC, which may contribute to ED improvement [19].
Despite its multifactorial nature, arousal disorders, secondary to vascular disease, seem to be important components of FSD. In females, arousal is accompanied by an increase in blood flow to genitalia, leading to vaginal lubrication and clitoral erection [9]. Cardiovascular diseases, including endothelial impairments and atherosclerotic processes have been implicated with reduced blood flow to female sexual organs, which may contribute to FSD. However, there are few studies addressing the relationship between innate immune system-induced inflammation and cell infiltration on FSD.
Triggers of inflammation
Pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs)
The recognition of pathogens by the innate immune system is mediated by pattern-recognition receptors (PRRs). PRRs are activated by pathogen-associated molecular patterns (PAMPs) derived from microbial DNA, lipoproteins, glycoproteins or microbial membrane components. Chronic infections, resulting from continuous PRR activation, have been associated with cardiovascular events [20]. The main PRRs are TLR, which are associated with the innate immune system and are responsible for triggering the inflammatory response. Lipopolysaccharides (LPS) are the major component of gram-negative bacterial wall and a potent TLR4 ligand. Elevation in the levels of LPS during septic shock results in an increase of pro-inflammatory cytokine production and endothelial dysfunction through TLR activation [21]. In addition, bacterial infections have been associated with an increase in peripheral arterial occlusive diseases, which may lead to reduced blood flow to genitourinary tract [22].
Damage-associated molecular patterns (DAMPs) are cell-derived endogenous molecules that can be released under tissue injury and initiate an inflammatory process through TLR activation [23]. Elevated levels of DAMPs have been associated with vascular and non-vascular diseases. Mitochondrial DNA and N-formyl peptide (F-MIT) are mitochondria-derived DAMPs that can act through TLR9 and formyl peptide receptor (FPR), respectively. Infusion of high levels of F-MIT into rats has been shown to result in hypotension as well as increased vascular permeability via FPR activation, highlighting the role of this pathway in vascular tone regulation [24,25]. Further, studies in spontaneously hypertensive rats (SHR) have shown that the elevation of circulating mitochondrial DNA contributed to vascular dysfunction and increased pressure levels through TLR9 activation [26]. Therefore, the activation of PRR by DAMPs and PAMPs is a pathway that triggers inflammation, which may contribute to smooth muscle and endothelial impairments and consequently sexual dysfunction (Figure 2 and 3).
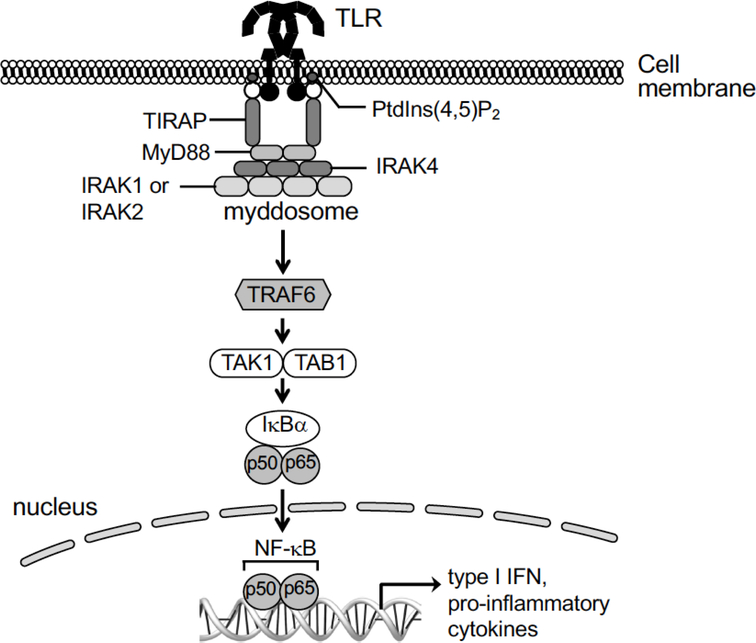
Figure 2.
Toll-like receptor signaling – general features of the canonical pathway. The myeloid differentiation primary response 88 (MyD88) is an innate immune signal transduction adaptor common for almost all TLR (except for the TLR3), which recruits interleukin 1 receptor-associated kinase (IRAK). The IRAK interacts with adapter protein tumor necrosis factor-associated factor 6 (TRAF6), leading to nuclear factor kappa light chain enhancer of activated B cells (NF-kB) translocation to the cellular nuclei. The TLRMyD88 signaling pathway results in increased pro-inflammatory cytokines and interferon production.
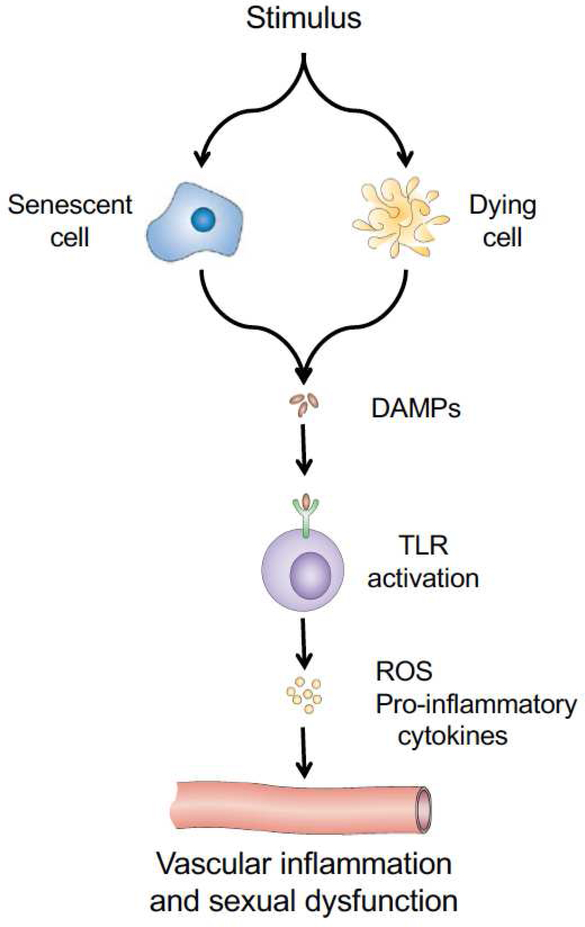
Figure 3.
Damage-associated molecular patterns (DAMPs) and vascular inflammation. DAMPs are released or secreted from dying or senescent cells, respectively. Once released, the DAMPs bind to TLRs leading to the production of reactive oxygen species and pro-inflammatory cytokines. These latter molecules act on the vasculature to cause inflammation and may contribute to penile or clitoral dysfunction.
Obesity, diabetes and hypertension
Obesity is one of the most important risk factor for cardiovascular events. It is associated with low-grade systemic inflammation, morphological changes in adipose tissue as well as increased number of immune cells and release of pro-inflammatory factors [27]. Inflammatory processes have been implicated in the pathophysiology of hypertension. In mice, the deficiency in IL-6 and TNF-α production attenuated the angiotensin II-induced hypertension [28]. Also, the inhibition of NF-kB prevented hypertension in SHR [29]. Recently, the cell damage present in hypertension has been shown to play a role in the inflammatory process through adaptive immune system activation [26]. In SHR, the increase in vascular TLR4 expression play a role in hypertension by a COX-dependent mechanism [30]. This vicious cycle between inflammation and hypertension can contribute for vascular dysfunction and chronic low-grade inflammation found in hypertension.
Obesity is also a risk factor for type 2 diabetes. The increase in free fatty acids (FFA) with obesity can lead to TLR4 activation that result in pro-inflammatory cytokine production and insulin resistance [31]. Interestingly, TLR4 knockout mice are protected from high-fat diet-induced obesity [32]. Diabetes-induced sexual dysfunction is considered multifactorial, including hormonal, neurogenic and metabolic impairments. The prevalence of ED in male diabetic patients ranges from 35 to 90%. Interestingly, diabetic men tend to develop ED 10 to 15 years before of the non-diabetic ED patients [33]. The prevalence of FSD in diabetic women is more heterogeneous, ranging from 12 to 88% [34].
Among the metabolic changes, the high blood glucose levels, which is considered a hallmark of diabetes, is implicated as an important factor in generating diabetes-associated vascular dysfunction through the inflammatory process. High glucose levels are able to up-regulate inflammatory genes in preadipocytes [35]. Hyperglycemia also results in high mobility group box 1 (HMGB1) protein release, which can increase cytokine and chemokine production through TLR activation [36]. Glucose can also alter gene expression and the phenotype profile of immune cells (e.g., monocytes and neutrophil). Interestingly, high glucose exacerbates FFA-induced monocyte-related inflammation via TLR activation [37]. Of note, hyperglycemia is associated with increased risk of genitourinary infections, which contributes to PAMP-associated inflammatory state.
Diabetes, obesity and hypertension can lead to vascular impairments in several arterial beds, including arteries responsible for arterial blood flow to the penis and clitoris. As stated before, decreased blood flow to sexual organs may contribute to sexual dysfunction. In fact, female SHR exhibit morphological changes in the clitoris that might be associated with reduced blood flow to the genitourinary tract [38]. Increases in serum endothelin-1 levels seen in hypertensive female rats can also contribute to FSD; endothelin receptor activation causes pudendal artery contraction, resulting in reduction of blood flow to clitoris and vagina [39]. Similarly, decreases in sexual response in female diabetic rats have been linked with an increase in pudendal artery contraction through ET-1/RhoA/Rho-kinase pathway [40]. Interestingly, TLR3 activation can increase ET-1 mRNA expression in human endothelial cells [41]. In males, the reduction of blood flow to the penis of SHR, due to vascular remodeling and increased α1-adrenoceptor expression on iliac and pudendal arteries, has been implicated with ED [42,43]. Pudendal arteries from male diabetic rats exhibited hypercontractility due to dysregulation of the arterial antioxidant system [44]. Further, the NO pathway exerts a pivotal role in male and female genitalia smooth muscle relaxation and genital blood flow. Studies have demonstrated reduced expression of NOS in low-grade inflammation-associated diseases [45]. Therefore, the chronic inflammatory state observed in obesity, diabetes and hypertension might be a link between vascular disease and sexual dysfunction (Figure 3).
Oxidized lipoproteins
Dyslipidemia is a common obesity-associated component, defined as hypertriglyceridemia, low levels of high-density lipoprotein (HDL) and high levels of low-density lipoprotein (LDL). The oxidation of LDL cholesterol seems to increase its atherogenic effect. Oxidized LDL molecules (OxLDL) are promptly taken up by macrophages, which can aggregate on vessels walls and are the major component of atherosclerotic lesions. Macrophages contribute to the local inflammatory state by secreting inflammatory enzymes (e.g. myeloperoxidase), cytokines (e.g. IL-6), and by attracting other immune cells (e.g. monocytes) [46]. Recently, it was demonstrated that OxLDL can also increase IL-1β and IL-18 levels through inflammasome and caspase-1 activation, suggesting that this pathway is a link between inflammation, lipid metabolism, and the atherosclerotic process [47]. Studies have demonstrated that high-density lipoprotein (HDL) is also susceptible to the oxidation process [48]. Oxidized HDL (OxHDL) exhibits cell proliferation and proinflammatory properties [49].
The activation of TLRs by OxLDL has been shown to play a pivotal role for the inflammatory response in the atherosclerotic process. It was demonstrated that TLR4 expression in macrophages is necessary for OxLDL-induced macrophage lipid accumulation and differentiation [50,51]. Interestingly, hypercholesterolemic TLR2 knockout mice are protected from atherosclerotic lesions [52]. Increased OxLDL levels in the corpus cavernosum have been clinically implicated with vascular erectile dysfunction [53]. In vitro incubation of rabbit corpus cavernosum with human OxLDL led to cavernosal smooth muscle hypercontractility [54]. Ischemia of female genitourinary tract, resulted in decreased clitorial smooth muscle cell content as well as vaginal and clitoral fibrosis in a rabbit model of atherosclerosis through iliac artery injury [55]. Similarly, OxHDL can also activate TLR4, leading to augmented endoplasmic reticulum stress and macrophage apoptosis, both of which contribute to the inflammation process and atherosclerotic lesions [56]. The role of dyslipidemia in the genesis of atherosclerotic process is already established. However, the chronic inflammatory nature involved on this process and its relationship with vascular and sexual dysfunction is still an open field for further studies.
Sex hormones
Immunological responses can vary between males and females, suggesting that sex hormones could play a role. It is well established that estrogen has a cardiovascular protective effect. The absence of estrogen has been implicated with endothelial dysfunction and increased serum levels of IL-6 and TNF-α in ovariectomized rats [57]. In human female endothelial cells derived from the iliac artery, pretreatment with estrogen decreased the IL-1β-induced vascular and intracellular cell adhesion molecule-1 (VCAM-1 and ICAM-1) expression, both molecules implicated in vascular inflammatory processes [58]. Interestingly, iliac arteries from postmenopausal women exhibited a thicker wall compared with reproductive women, which can also contribute to vasculogenic FSD [59]. However, estradiol seems to have a dual effect on pro-inflammatory cytokine production. At low doses, estradiol increases the production of pro-inflammatory cytokines (IL-6 and TNF-α) in human macrophages while high doses have the opposite effect [60].
It has been reported that androgen hormones exhibit an immunosuppressive effect, acting on cellular components of the innate immune system. Testosterone has been shown to decrease the expression of TLR4 and reduce the synthesis of TNF-α and iNOS in macrophages after in vitro and in vivo exposure [61]. In human males, androgen deficiency leads to increases in serum inflammatory cytokine levels (e.g., IL1-β and TNF-α) and the number of macrophages in the circulation [62]. Reduced androgens levels also resulted in vascular dysfunction characterized by iliac arterial fibrosis and remodeling in castrated rats [63]. Testosterone can dilate iliac arteries from males through a NO-dependent mechanism, which may contribute to increased blood flow to the genitourinary tract [64]. Androgen replacement in type 2 diabetic rats reduced the inflammatory process in CC, improving ED [18].
It is important to note that androgens have been shown to play an important role in the female genitourinary tract [65,66]. The intravaginal administration of desidroepiandrosterone (DHEA) reduces dyspareunia, by increasing lubrication leading to better satisfaction in postmenopausal women [67,68]. Likewise, testosterone replacement therapy has been used clinically to counterbalance the reduced sexual desire seen in postmenopausal women [69]. The pathway by which androgens ameliorate FSD is still a matter of debate, however it is possible that a reduced inflammatory response may play a role on this process.
Therefore, despite the concentration-dependent dual effect of estrogen observed in human macrophages, studies have demonstrated that deprivation of androgens and estrogen can positively modulate the inflammatory response in vascular and cavernosal tissue, contributing to male and FSD.
Sexual dysfunction predicts CVD
Cardiovascular diseases comprise heart and blood vessel impairments due to vessel narrowing or blockage and may result in heart attacks, stroke, and peripheral vascular disease. The development of these diseases occurs through endothelium damage and show strong relationships with increased inflammation and activation of the immune system [70]. For instance, studies involving human patients have shown elevated levels of inflammatory cytokines, cell adhesion proteins, acute-phase proteins, and lipid factors to be predictive markers of CVD development [71–73].
Epidemiological data support the association between sexual dysfunction and CVD. In hypertensive women, FSD ranges from 22 to 49% [74,34]. Likewise, 33 to 60% of women with coronary heart disease experienced at least one symptom related to FSD [74]. In men, the CVD-related ED is well established and the prevalence is higher than 70% in patients that exhibit some cardiovascular dysfunction such as coronary heart disease, stroke and peripheral artery disease [75].
Experimental and human evidence show that ED may occur in patients prior to the occurrence of CVD. Rats fed with a western diet exhibited impairment in erectile function after 8 weeks, while coronary endothelial dysfunction was present only after 12 weeks [76]. Similarly, in type 1 diabetic mice, impaired cell-to-cell junctions were exhibited in cavernous sinusoid endothelium but not in the coronary artery or femoral artery [77]. This phenomenon could be explained by the “artery size hypothesis”, which suggests that blood flow to arteries of the penis will be compromised by atherosclerotic plaques earlier than other vessels due to their smaller diameter [78]. Asian Indians reported to have ED symptoms 2 years prior to symptoms of coronary artery disease (CAD) and the severity of ED was positively correlated with the number of compromised coronary vessels observed by angiography [79]. Accordingly, studies reported the occurrence of ED symptoms prior to CAD symptoms in a timeframe varying between 2–5 years [80,81].
There are several inflammatory predictive markers of CVD that have also been implicated in male and FSD. Together, this is suggestive that both inflammation and ED might be earlier markers for CVD. Thus, the development of sexual dysfunction can be used as a predicted “window” into total cardiovascular health for both genders, although FSD is less studied.
Vascular inflammation and sexual dysfunction – evidence from humans
As previously stated, hypertension, obesity, and diabetes are conditions that are closely related to vascular inflammation and endothelial dysfunction. The low-grade inflammatory process seen in hypertensive patients affects endothelial and vascular smooth muscle cells and may contribute to accelerated vascular aging [82]. Clinically, hypertensive patients exhibit higher levels of serum pro-inflammatory cytokines (e.g. IL-6 and TNF-α) compared with normotensive patients [83]. Elevated serum levels of inflammatory markers, such as IL-6, have been correlated with ED in men [14]. Interestingly, the association between sexual dysfunction and cardiovascular risk factors seems to be milder in females than in males; however, this could be due to understudied FSD [84].
In obese patients, increased serum levels of TNF-α and pro-inflammatory cytokine gene expression were found in monocytes and lymphocytes [85]. Moreover, mast cells from obese patients produce higher levels of pro-inflammatory cytokines (IL-1β, IL-6 and IL-16) and chemokines (CCL2, CXCL1 and CXCL8) compared to lean subjects [27]. In type 2 diabetic patients from both sexes, iliac arteries exhibited subclinical arterial inflammation, which may be associated with arterial stiffness [86]. TLR expression is increased in monocytes from male and female diabetic patients compared with non-diabetic group, further contributing to innate immune system-induced inflammatory response and vascular dysfunction [87].
Retinal blood flow, periodontitis and sexual dysfunction
Reduction in retinal blood flow due to microvascular dysfunction has been shown to be a predictive factor of CVD [88]. Similarly, studies have demonstrated the relationship between retinal vascular impairments as early signs for ED. Clinically, abnormal retinal vascular findings were observed in 63.3% of the patients with arteriogenic ED, while only 33.3% of non-arteriogenic ED patients exhibited retinal vascular abnormalities [89]. It was also demonstrated that reductions in the peak systolic velocity of blood flow to CC is correlated with retinal vascular stenosis in patients with ED [90]. Reductions in retinal arteriolar caliber was also positively associated with ED in type 2 diabetic patients [91].
Periodontal disease has also been associated with endothelial dysfunction, however, there are only a few clinical studies addressing the relationship between periodontitis and ED [92]. In fact, the prevalence of periodontitis in patients with severe vasculogenic ED is estimated to be 81.8% [93]. Further, two other studies have found higher prevalence of periodontal disease among men with mild to severe ED [94,95].
The exact mechanism by which periodontitis could be related to ED is still unclear. Studies have shown that periodontitis increases systemic inflammation in hypertensive and obese patients [96,97]. The presence of periodontitis and peripheral arterial disease was associated with increased serum levels of IL-6 and TNF-α. Similarly, the prevalence of periodontitis is higher in patients diagnosed with aorto-iliac occlusive disease compared with controls. Moreover, patients who exhibited periodontitis had a 5-fold increased chance of developing peripheral arterial disease than periodontally healthy subjects [98]. In agreement, another study has shown that more than 50% of patients diagnosed with peripheral arterial disease exhibit severe periodontitis [99]. To the best of our knowledge, there is no clinical study addressing specifically the role of periodontitis and blood flow to male and female sexual organs. However, since the above-cited studies have shown the relationship between periodontitis and systemic inflammation, endothelial dysfunction and ED, it is plausible to consider the negative vascular impact of periodontitis in penile and clitoral blood flow.
Pharmacological evidence
Taking in consideration the close relationship between ED and CVD, it should be expected that drugs used for the treatment of CVD might be a useful tool for the management of ED. However, it is interesting to note that some anti-hypertensive drugs failed to restore or had a detrimental effect on sexual function in male hypertensive patients [100]. Although the first line of ED treatment are PDE5 inhibitors and despite of its effects/benefits for the cardiovascular system, it is important to be aware of the interactions between PDE5 inhibitors and drugs used to treat CVD [101]. Because ED and CVD usually coexist, it might be of interest to develop drugs capable of managing both conditions and thus, the inhibition of inflammatory state might be considered a valuable therapeutic target.
Although earlier studies show that the use of non-steroidal anti-inflammatory drugs (NSAID) was related to ED development, a recent cohort study showed only a modest correlation between NSAID use and ED, which is more compatible with the inflammatory hypothesis of ED [102]. To date, animal studies have reported improvement in the erectile function after treatment with drugs presenting anti-inflammatory properties or inhibition of innate immune system resulting in decreased inflammation [103]. In humans, a recent study revealed improvement in the sexual function of males and females treated with a combination of lenalidomide and dexamethasone in patients with sexual dysfunction caused by hypogonadism [104]. Therefore, there are few studies correlating the innate immune system and inflammation to the physiopathology of ED and more evidence is necessary as it could be a valuable avenue and a novel therapeutic target for ED.
Conclusion
Short-term activation of innate and adaptive systems are crucial for the immune system to exert its protective effects. However, sustained activation may lead to tissue damage and a chronic inflammatory state that is associated with several diseases such as hypertension, obesity and diabetes. The above-cited studies suggest a role of the immune system, through TLR-induced pro-inflammatory cytokine production and immune cell tissue infiltration, in generating an inflammatory environment, which contributes to vascular impairments and FSD/ED development. Therefore, targeting the immune system could be a valuable target to improve sexual dysfunction by reducing vascular and systemic inflammation.
Acknowledgments
Funding: This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant number 2016/20592–8) and the National Institutes of Health (HL-134604).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lotti F, Maggi M. Sexual dysfunction and male infertility. Nat Rev Urol 2018;15(5):287–307. [DOI] [PubMed] [Google Scholar]
- [2].Lewis RW, Fugl-Meyer KS, Corona G, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med 2010;7(4 Pt 2):1598–607. [DOI] [PubMed] [Google Scholar]
- [3].Rodrigues FL, Fais RS, Tostes RC et al. There is a link between erectile dysfunction and heart failure: it could be inflammation. Curr Drug Target 2015; 16(5):442–450. [DOI] [PubMed] [Google Scholar]
- [4].Maiorino MI, Bellastella G, Giugliano D, et al. From inflammation to sexual dysfunctions: a journey through diabetes, obesity, and metabolic syndrome. J Endocrinol Invest 2018; 41(11):1249–1258. [DOI] [PubMed] [Google Scholar]
- [5].Puppo V, Puppo G. Anatomy of sex: Revision of the new anatomical terms used for the clitoris and the female orgasm by sexologists. Clin Anat 2015;28(3):293–304. [DOI] [PubMed] [Google Scholar]
- [6].Hannan JL, Cheung GL, Blaser MC, et al. Characterization of the vasculature supplying the genital tissues in female rats. J Sex Med 2012;9(1):136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brackett NL, Lynne CM, Ibrahim E, et al. Treatment of infertility in men with spinal cord injury. Nat Rev Urol 2010;7(3):162–172. [DOI] [PubMed] [Google Scholar]
- [8].O’Connell HE, Eizenberg N, Rahman M, et al. The anatomy of the distal vagina: towards unity. J Sex Med 2008;5(8):1883–91. [DOI] [PubMed] [Google Scholar]
- [9].Maravilla KR, Yang CC. Magnetic resonance imaging and the female sexual response: overview of techniques, results, and future directions. J Sex Med 2008;5(7):1559–71. [DOI] [PubMed] [Google Scholar]
- [10].Bush PA, Aronson WJ, Buga GM, et al. Nitric oxide is a potent relaxant of human and rabbit corpus cavernosum. J Urol 1992;147(6):1650–5. [DOI] [PubMed] [Google Scholar]
- [11].Andersson KE. PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol 2018;175(13):2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burnett AL, Calvin DC, Silver RI, et al. Immunohistochemical description of nitric oxide synthase isoforms in human clitoris. J Urol 1997;158(1):75–8. [DOI] [PubMed] [Google Scholar]
- [13].Krassioukov A, Elliott S. Neural Control and Physiology of Sexual Function: Effect of Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2017; 23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vlachopoulos C, Aznaouridis K, Ioakeimidis N, et al. Unfavourable endothelial and inflammatory state in erectile dysfunction patients with or without coronary artery disease. Eur Heart J 2006;27(22):2640–8. [DOI] [PubMed] [Google Scholar]
- [15].Carneiro FS, Zemse S, Giachini FR, et al. TNF-alpha infusion impairs corpora cavernosa reactivity. J Sex Med 2009;6 Suppl 3:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nunes KP, Bomfim GF, Toque HA, et al. Toll-like receptor 4 (TLR4) impairs nitric oxide contributing to Angiotensin II-induced cavernosal dysfunction. Life Sci 2017;191:219–226. [DOI] [PubMed] [Google Scholar]
- [17].Matsui H, Sopko NA, Hannan JL, et al. M1 Macrophages Are Predominantly Recruited to the Major Pelvic Ganglion of the Rat Following Cavernous Nerve Injury. J Sex Med 2017;14(2):187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kataoka T, Hotta Y, Maeda Y, et al. Assessment of androgen replacement therapy for erectile function in rats with type 2 diabetes mellitus by examining nitric oxide-related and inflammatory factors. J Sex Med 2014;11(4):920–9. [DOI] [PubMed] [Google Scholar]
- [19].Vlachopoulos C, Ioakeimidis N, Rokkas K, et al. Acute effect of sildenafil on inflammatory markers/mediators in patients with vasculogenic erectile dysfunction. Int J Cardiol 2015;182:98–101. [DOI] [PubMed] [Google Scholar]
- [20].Szeto CC, McIntyre CW, Li PK. Circulating Bacterial Fragments as Cardiovascular Risk Factors in CKD. J Am Soc Nephrol 2018;29(6):1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ding J, Song D, Ye X, et al. A pivotal role of endothelial-specific NF-kappaB signaling in the pathogenesis of septic shock and septic vascular dysfunction. J Immunol 2009;183(6):4031–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Linares-Palomino JP, Gutierrez J, Lopez-Espada C, et al. Genomic, serologic, and clinical case-control study of Chlamydia pneumoniae and peripheral artery occlusive disease. J Vasc Surg 2004;40(2):359–66. [DOI] [PubMed] [Google Scholar]
- [23].Matzinger P The danger model: a renewed sense of self. Science 2002. 296(5566):301–5. [DOI] [PubMed] [Google Scholar]
- [24].Wenceslau CF, McCarthy CG, Szasz T, et al. Mitochondrial N-formyl peptides induce cardiovascular collapse and sepsis-like syndrome. Am J Physiol Phys Circ 2015;308 (7):H768–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wenceslau CF, McCarthy CG, Szasz T, et al. Formyl peptide receptor-1 activation exerts a critical role for the dynamic plasticity of arteries via actin polymerization. Phar Res 2019;141:276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McCarthy CG, Wenceslau CF, Goulopoulou S, et al. Circulating mitochondrial DNA and Toll-like receptor 9 are associated with vascular dysfunction in spontaneously hypertensive rats. Cardiovasc Res 2015;107(1):119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zelechowska P, Agier J, Kozlowska E, et al. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes Rev 2018;19(5):686–69. [DOI] [PubMed] [Google Scholar]
- [28].Sriramula S, Haque M, Majid DS, et al. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension 2008;51(5):1345–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rodriguez-Iturbe B, Ferrebuz A, Vanegas V, et al. Early and sustained inhibition of nuclear factor-kappaB prevents hypertension in spontaneously hypertensive rats. J Pharmacol Exp Ther 2005;315(1):51–7. [DOI] [PubMed] [Google Scholar]
- [30].Bomfim GF, Dos Santos RA, Oliveira MA, et al. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci (Lond) 2012;122(11):535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116(11):3015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pierre N, Deldicque L, Barbe C, et al. Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS One 2013;8(5):e65061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Calogero AE, Burgio G, Condorelli RA, et al. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male 2019; 22(1):12–19. [DOI] [PubMed] [Google Scholar]
- [34].Maseroli E, Scavello I, Vignozzi L. Cardiometabolic Risk and Female Sexuality-Part I. Risk Factors and Potential Pathophysiological Underpinnings for Female Vasculogenic Sexual Dysfunction Syndromes. Sex Med Rev 2018; 6(4):508–524. [DOI] [PubMed] [Google Scholar]
- [35].Ronningen T, Shah A, Reiner AH, et al. Epigenetic priming of inflammatory response genes by high glucose in adipose progenitor cells. Biochem Biophys Res Commun 2015;467(4):979–86. [DOI] [PubMed] [Google Scholar]
- [36].Chu Y, Wang Y, Zheng Z, et al. Proinflammatory Effect of High Glucose Concentrations on HMrSV5 Cells via the Autocrine Effect of HMGB1. Fronti Physiol 2017;8: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Meatb 2011;300(1):E145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bechara AJ, Cao G, Casabe AR, et al. Morphological modifications in clitoris and vagina in spontaneously hypertensive rats. Int J Impot Res 2003;15(3):166–72. [DOI] [PubMed] [Google Scholar]
- [39].Allahdadi KJ, Hannan JL, Tostes RC, et al. Endothelin-1 induces contraction of female rat internal pudendal and clitoral arteries through ET(A) receptor and rho-kinase activation. J Sex Med 2010;7(6):2096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Allahdadi KJ, Hannan JL, Ergul A, et al. Internal pudendal artery from type 2 diabetic female rats demonstrate elevated endothelin-1-mediated constriction. J Sex Med 2011;8(9):2472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Farina G, York M, Collins C, et al. dsRNA activation of endothelin-1 and markers of vascular activation in endothelial cells and fibroblasts. Ann Rheum Dis 2011;70(3):544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yono M, Tanaka T, Tsuji S, et al. Effects of age and hypertension on alpha1-adrenoceptors in the major source arteries of the rat bladder and penis. Eur J Pharmacol 2011;670(1):260–5. [DOI] [PubMed] [Google Scholar]
- [43].Hannan JL, Blaser MC, Pang JJ, et al. Impact of hypertension, aging, and antihypertensive treatment on the morphology of the pudendal artery. J Sex Med 2011;8(4):1027–38. [DOI] [PubMed] [Google Scholar]
- [44].Alves-Lopes R, Neves KB, Montezano AC, et al. Internal Pudental Artery Dysfunction in Diabetes Mellitus Is Mediated by NOX1-Derived ROS-, Nrf2-, and Rho Kinase-Dependent Mechanisms. Hypertension 2016;68(4):1056–64. [DOI] [PubMed] [Google Scholar]
- [45].Valerio A, Cardile A, Cozzi V, et al. TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 2006;116(10):2791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Heinecke JW. Lipoprotein oxidation in cardiovascular disease: chief culprit or innocent bystander? J Exp Med 2006;203(4):813–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med 2015;21(7):677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, et al. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Progr Lipid Res 2012;51(4):314–24. [DOI] [PubMed] [Google Scholar]
- [49].Wang Y, Ji L, Jiang R, et al. Oxidized high-density lipoprotein induces the proliferation and migration of vascular smooth muscle cells by promoting the production of ROS. J Atheroscler Thromb 2014;21(3):204–16. [DOI] [PubMed] [Google Scholar]
- [50].Howell KW, Meng X, Fullerton DA, et al. Toll-like receptor 4 mediates oxidized LDL-induced macrophage differentiation to foam cells. J Surg Res 2011;171(1):e27–31. [DOI] [PubMed] [Google Scholar]
- [51].Yang K, Wang X, Liu Z, et al. Oxidized low-density lipoprotein promotes macrophage lipid accumulation via the toll-like receptor 4-Src pathway. Circ J 2015;79(11):2509–16. [DOI] [PubMed] [Google Scholar]
- [52].Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest 2005;115(11):3149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zouaoui Boudjeltia K, Roumeguere T, Delree P, et al. Presence of LDL modified by myeloperoxidase in the penis in patients with vascular erectile dysfunction: a preliminary study. Eur Urol 2007;51(1): 262–8. [DOI] [PubMed] [Google Scholar]
- [54].Ahn TY, Gomez-Coronado D, Martinez V, et al. Enhanced contractility of rabbit corpus cavernosum smooth muscle by oxidized low density lipoproteins. Int J Impot Res 1999;11(1):9–14. [DOI] [PubMed] [Google Scholar]
- [55].Park K, Tarcan T, Goldstein I, et al. Atherosclerosis-induced chronic arterial insufficiency causes clitoral cavernosal fibrosis in the rabbit. In t J Impot Res 2000;12(2):111–6. [DOI] [PubMed] [Google Scholar]
- [56].Yao S, Tian H, Zhao L, et al. Oxidized high density lipoprotein induces macrophage apoptosis via toll-like receptor 4-dependent CHOP pathway. J Lipid Res 2017;58(1):164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lamas AZ, Caliman IF, Dalpiaz PL, et al. Comparative effects of estrogen, raloxifene and tamoxifen on endothelial dysfunction, inflammatory markers and oxidative stress in ovariectomized rats. Life Sci 2015;124:101–9. [DOI] [PubMed] [Google Scholar]
- [58].Piercy KT, Donnell RL, Kirkpatrick SS, et al. Effects of estrogen, progesterone, and combination exposure on interleukin-1 beta-induced expression of VCAM-1, ICAM-1, PECAM, and E-selectin by human female iliac artery endothelial cells. J Surg Res 2002;105(2):215–9. [DOI] [PubMed] [Google Scholar]
- [59].Baron YM, Brincat M, Galea R. Iliac vessel wall thickness in menstrual and hormone treated and untreated postmenopausal women. Gynecol Endocrinol 2012;28(5):409–12. [DOI] [PubMed] [Google Scholar]
- [60].Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005;11(4):411–23. [DOI] [PubMed] [Google Scholar]
- [61].D’Agostino P, Milano S, Barbera C, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y Acad Sci 1999;876:426–9. [DOI] [PubMed] [Google Scholar]
- [62].Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol 2010;73(5):602–12. [DOI] [PubMed] [Google Scholar]
- [63].Magari T, Shibata Y, Arai S, et al. Time-dependent effects of castration on the bladder function and histological changes in the bladder and blood vessels. Asian J Androl 2014;16(3):457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Molinari C, Battaglia A, Grossini E, et al. The effect of testosterone on regional blood flow in prepubertal anaesthetized pigs. J Physiol 2002;543(Pt 1):365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Traish AM, Vignozzi L, Simon JA, et al. Role of Androgens in Female Genitourinary Tissue Structure and Function: Implications in the Genitourinary Syndrome of Menopause. Sex Med Rev 2018; 6(4):558–571. [DOI] [PubMed] [Google Scholar]
- [66].Bonilla-Becerra SM, de Oliveira MG, Calmasini FB, et al. Micturition dysfunction in four-month old ovariectomized rats: Effects of testosterone replacement. Life Sci 2017; 179:120–129. [DOI] [PubMed] [Google Scholar]
- [67].Labrie F, Derogatis L, Archer DF, et al. Effect of Intravaginal Prasterone on Sexual Dysfunction in Postmenopausal Women with Vulvovaginal Atrophy. J Sex Med 2015; 12(12):2401–2412. [DOI] [PubMed] [Google Scholar]
- [68].Simon JA, Goldstein I, Kim NN, et al. The role of androgens in the treatment of genitourinary syndrome of menopause (GSM): International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Menopause 2018; 25(7):837–847. [DOI] [PubMed] [Google Scholar]
- [69].Davis SR, Worsley R. Androgen treatment of postmenopausal women. J Steroid Biochem Mol Biol 2014; 142:107–114. [DOI] [PubMed] [Google Scholar]
- [70].Li H, Cybulsky MI, Gimbrone MA, et al. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb 1993;13(2):197–204. [DOI] [PubMed] [Google Scholar]
- [71].Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Eng J Med 2000;342(12):836–43. [DOI] [PubMed] [Google Scholar]
- [72].Ridker PM, Rifai N, Pfeffer M, et al. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000;101(18):2149–53. [DOI] [PubMed] [Google Scholar]
- [73].Hwang SJ, Ballantyne CM, Sharrett AR, et al. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation 1997;96(12):4219–25. [DOI] [PubMed] [Google Scholar]
- [74].Miner M, Esposito K, Guay A, et al. Cardiometabolic risk and female sexual health: the Princeton III summary. J Sex Med 2012; 9(3):641–651. [DOI] [PubMed] [Google Scholar]
- [75].Katsiki N, Wierzbicki AS, Mikhailidis DP. Erectile dysfunction and coronary heart disease. Curr Opin Cardiol 2015; 30(4):416–421. [DOI] [PubMed] [Google Scholar]
- [76].La Favor JD, Anderson EJ, Hickner RC, et al. Erectile dysfunction precedes coronary artery endothelial dysfunction in rats fed a high-fat, high-sucrose, Western pattern diet. J Sex Med 2013;10(3):694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ryu JK, Jin HR, Yin GN, et al. Erectile dysfunction precedes other systemic vascular diseases due to incompetent cavernous endothelial cell-cell junctions. J Urol 2013;190(2):779–89. [DOI] [PubMed] [Google Scholar]
- [78].Montorsi P, Ravagnani PM, Galli S, et al. The artery size hypothesis: a macrovascular link between erectile dysfunction and coronary artery disease. Am J Cardiol 2005;96(12B): 19M–23M. [DOI] [PubMed] [Google Scholar]
- [79].Kumar J, Bhatia T, Kapoor A, et al. Erectile dysfunction precedes and is associated with severity of coronary artery disease among Asian Indians. J Sex Med 2013;10(5):1372–9. [DOI] [PubMed] [Google Scholar]
- [80].Thompson IM, Tangen CM, Goodman PJ, et al. Erectile dysfunction and subsequent cardiovascular disease. JAMA 2005;294(23):2996–3002. [DOI] [PubMed] [Google Scholar]
- [81].Andrade WS, Oliveira P, Laydner H, et al. Severity of erectile dysfunction is highly correlated with the syntax score in patients undergoing coronariography. Int Braz J Urol 2016;42(1):123–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Barton M, Husmann M, Meyer MR. Accelerated Vascular Aging as a Paradigm for Hypertensive Vascular Disease: Prevention and Therapy. Can J Cardiol 2016;32(5):680–6 e4. [DOI] [PubMed] [Google Scholar]
- [83].Bautista LE, Vera LM, Arenas IA, et al. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hyperten 2005;19(2):149–54. [DOI] [PubMed] [Google Scholar]
- [84].Maseroli E, Scavello I, Vignozzi L. Cardiometabolic Risk and Female Sexuality-Part I. Risk Factors and Potential Pathophysiological Underpinnings for Female Vasculogenic Sexual Dysfunction Syndromes. Sex Med Rev 2018;6(4):508–524. [DOI] [PubMed] [Google Scholar]
- [85].Hong SB, Lee JJ, Kim SH, Suh YJ, Han JY, Kim YS, et al. The effects of adiponectin and inflammatory cytokines on diabetic vascular complications in obese and non-obese patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 2016;111:58–65. [DOI] [PubMed] [Google Scholar]
- [86].de Boer SA, Hovinga-de Boer MC, Heerspink HJ, et al. Arterial Stiffness Is Positively Associated With 18F-fluorodeoxyglucose Positron Emission Tomography-Assessed Subclinical Vascular Inflammation in People With Early Type 2 Diabetes. Diabetes Care 2016;39(8):1440–7. [DOI] [PubMed] [Google Scholar]
- [87].Gupta S, Maratha A, Siednienko J, Natarajan A, Gajanayake T, Hoashi S, et al. Analysis of inflammatory cytokine and TLR expression levels in Type 2 Diabetes with complications. Sci Rep 2017;7(1):7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Al-Fiadh AH, Wong TY, Kawasaki R, et al. Usefulness of retinal microvascular endothelial dysfunction as a predictor of coronary artery disease. Am J Cardiol 2015;115(5):609–13. [DOI] [PubMed] [Google Scholar]
- [89].Emarah AM, El-Haggar SM, Osman IA, et al. Correlation between penile cavernosal artery blood flow and retinal vascular findings in arteriogenic erectile dysfunction. Clin Ophthalmol 2010;4:1047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kawanishi Y, Kimura K, Nakanishi R, et al. Retinal vascular findings and penile cavernosal artery blood flow. BJU Int 2003;92(9):977–80. [DOI] [PubMed] [Google Scholar]
- [91].Chew SK, Taouk Y, Xie J, et al. The relationship of retinal vessel caliber with erectile dysfunction in patients with type 2 diabetes. Invest Ophthalmol Vis Sci 2013;54(12):7234–9. [DOI] [PubMed] [Google Scholar]
- [92].Piconi S, Trabattoni D, Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J 2009;23(4):1196–204. [DOI] [PubMed] [Google Scholar]
- [93].Sharma A, Pradeep AR, Raju PA. Association between chronic periodontitis and vasculogenic erectile dysfunction. J Periodont 2011;82(12):1665–9. [DOI] [PubMed] [Google Scholar]
- [94].Zadik Y, Bechor R, Galor S, et al. Erectile dysfunction might be associated with chronic periodontal disease: two ends of the cardiovascular spectrum. J Sex Med 2009;6(4):1111–6. [DOI] [PubMed] [Google Scholar]
- [95].Oguz F, Eltas A, Beytur A, et al. Is there a relationship between chronic periodontitis and erectile dysfunction? J Sex Med 2013;10(3):838–43. [DOI] [PubMed] [Google Scholar]
- [96].Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. N Eng J Med 2007;356(9):911–20. [DOI] [PubMed] [Google Scholar]
- [97].Nibali L, Tatarakis N, Needleman I, et al. Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J CLin Endocrinol Metab 2013;98(3):913–20. [DOI] [PubMed] [Google Scholar]
- [98].Chen YW, Umeda M, Nagasawa T, et al. Periodontitis may increase the risk of peripheral arterial disease. Eur J Vasc Endovasc Surg 2008;35(2):153–8. [DOI] [PubMed] [Google Scholar]
- [99].Igari K, Inoue Y, Iwai T. The Epidemiologic and Clinical Findings of Patients with Buerger Disease. Ann Vasc Surg 2016;30:263–9. [DOI] [PubMed] [Google Scholar]
- [100].Imprialos KP, Stavropoulos K, Doumas M, et al. Sexual Dysfunction, Cardiovascular Risk and Effects of Pharmacotherapy. Curr Vasc Pharmacol 2018;16(2):130–42. [DOI] [PubMed] [Google Scholar]
- [101].Corinaldesi C, Di Luigi L, Lenzi A, et al. Phosphodiesterase type 5 inhibitors: back and forward from cardiac indications. J Endocrinol Invest 2016;39(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Gleason JM, Slezak JM, Jung H, et al. Regular nonsteroidal anti-inflammatory drug use and erectile dysfunction. J Urol 2011;185(4):1388–93. [DOI] [PubMed] [Google Scholar]
- [103].Facio FN Jr., Facio MF, Spessoto LF, et al. Anti-inflammatory and anti-fibrotic effects of annexin1 on erectile function after cavernous nerve injury in rats. Int J Impot Res 2016;28(6):221–7. [DOI] [PubMed] [Google Scholar]
- [104].Yang H, Huang X, Cai Q, et al. Improvement of sexual function in POEMS syndrome after combination therapy of Lenalidomide and dexamethasone. Orphanet J Rare Dis 2016;11(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]