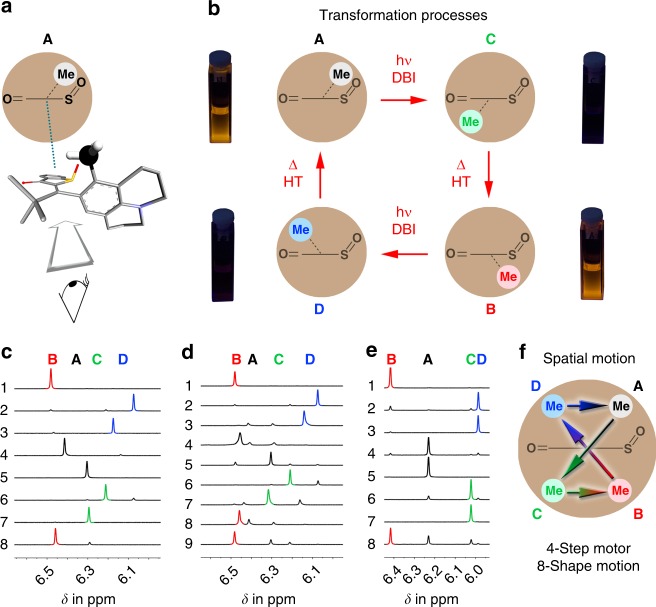
Fig. 3.
Molecular motor properties of 1. a Schematic representation of the geometry of A. b Four-step process of the motor motion and associated fluorescence changes. c Individual steps of motor operation followed by 1H NMR spectroscopy, (1) B in cyclohexane-d12, (2) after 520 nm irradiation, (3) D in acetonitrile-d3/D2O (8/2), (4) after heating to 27 °C, (5) A in cyclohexane-d12, (6) after 520 nm irradiation, (7) C in acetonitrile-d3/D2O (8/2), (8) after heating to 60 °C. d One full cycle of motor operation followed by 1H NMR spectroscopy, (1) B in cyclohexane-d12, (2) after 520 nm irradiation, (3) after solvent change to acetonitrile-d3/D2O (8/2), (4) after heating to 60 °C, (5) after solvent change to cyclohexane-d12, (6) after irradiation with 520 nm, (7) after solvent change to acetonitrile-d3/D2O (8/2), (8) after heating to 60 °C, (9) after solvent change to cyclohexane-d12. e Individual steps of autonomous motor operation followed by 1H NMR spectroscopy in 12DCB-d4, (1) pure B, (2) after 520 nm irradiation, (3) pure D, (4) after heating to 130 °C, (5) pure A, (6) after 520 nm irradiation, (7) pure C, (8) after heating to 130 °C. f Positional changes of the methyl group with respect to the static thioindigo fragment during one full cycle of directional motion