Abstract
Sex differences in empathy for pain have been repeatedly observed. However, it is unclear whether this is due to sex differences in “bottom-up” somatomotor representations of others’ pain (self-other resonance) or to “top-down” prefrontal control of such responses. Here we provide data from 70 subjects suggesting that sex differences in empathy for pain lie primarily in pre-reflective, bottom-up resonance mechanisms. Subjects viewed a right hand pierced by a needle during fMRI. They also filled out a self-report measure of trait empathy, the Interpersonal Reactivity Index. A permutation-based analysis (FSL’s Randomise) found that females showed greater signal in a cluster in primary somatomotor cortex that includes the motor hand area. No significant differences were observed in other task-implicated areas. An examination of condition-specific parameter estimates found that this difference was due to reduced signal in this cluster in males. No significant differences in resting connectivity or within-task (generalized psychophysiological interaction analysis or gPPI) dynamic connectivity of this region with prefrontal areas were observed. While female subjects scored higher on affective subscales of the IRI, there were no sex differences in Perspective-Taking, the primary index of cognitive, top-down empathy processes. These findings suggest that localized internal somatomotor representations of others’ pain, a functional index of bottom-up resonance processes, are stronger in female subjects.
Keywords: Sex, pain, empathy, functional magnetic resonance imaging, cognitive neuroscience
There is mounting evidence that sex differences in normal and pathological brain function are widespread and important, yet they remain poorly understood (McCarthy et al., 2012). This is particularly relevant in the domain of empathy (Christov-Moore et al., 2014). Empathy is a cornerstone of prosocial behavior in social animals. It allows us to experience the internal states of others and informs our conscious inferences about others’ thoughts and intentions. An important, reflexive subcomponent of empathy is self-other resonance (Christov-Moore and Iacoboni 2016a){henceforth “resonance”}, our ability to process the affective, somatosensory and behavioral states of others almost as if they were our own. This ability, known also as experience sharing (Zaki and Ochsner 2012) or mirroring (Iacoboni 2009), has been linked to self-reported trait empathy (Avenanti et al. 2009; Pfeifer et al. 2008; Singer et al. 2004), moral decision-making (Christov-Moore et al. 2017) as well as prosocial behavior in daily life (Morelli et al. 2014), and costly altruism (Christov-Moore and Iacoboni 2016a). Alterations in resonance have been associated with psychopathy (Nicholls and Petrila 2005), schizophrenia (Häfner, 2003) and autism spectrum disorder (ASD) (Dapretto et al. 2005; Halladay et al. 2015). Interventions aimed at behaviors associated with resonance, such as imitation, have been shown to improve social cognition in ASD (Ingersoll and Schreibman 2006). This suggests that resonance processes are a key factor in empathic functioning, important for social competence and relevant to mental health.
In the case of pain, observing the pain of others (or being made aware that another is in pain) has been found to activate systems for affective and somatosensory processing typically associated with experiencing pain (Avenanti et al. 2009; Christov-Moore and Iacoboni 2016a; Hein et al. 2010; Lamm et al. 2011). The nature and extent of this vicarious activation depends greatly on the properties of the stimuli and the experimental conditions (Lamm et al. 2011), as well as the context of the exposure.
Indeed, although resonance processes are thought to be pre-reflective, they are modulated by contextual factors such as social distance, status, group affiliation and perceived trustworthiness (Cheng et al. 2010; Gu and Han 2007; Guo et al. 2012; Hein and Singer 2008; Lamm et al. 2007; Loggia et al. 2008; Singer et al. 2006). This modulation seems a) implicit, given the pre-reflective nature of resonance (Christov-Moore and Iacoboni 2016a), b) inhibitory (Christov-Moore et al. 2016b), and c) persistent: Prefrontal cortex lesions associated with compulsive imitative behavior suggest that some mechanisms to control resonance are always at play, unless damaged (Lhermitte 1983; De Renzi et al. 1996). This sensitivity to context is likely the result of top-down control processes carried out by control systems that include medial and dorsolateral prefrontal cortex (MPFC and DLPFC) and perhaps the temporoparietal junction (TPJ) (Banks et al. 2007; Brighina et al. 2010; Cho and Strafella 2009; Cross et al. 2013; Decety and Lamm 2007; Miller and Cohen 2001; Spengler et al. 2009, 2010, Tassy et al. 2012; Volman et al. 2011; Winecoff et al. 2013). In line with these findings, we have recently proposed that resonance and control processes may exist in dynamic interaction during daily life (Christov-Moore and Iacoboni 2016a; Christov-Moore et al. 2017).
There is a great deal of evidence for sex differences in empathy and associated brain function (Christov-Moore et al., 2014). Females are frequently more empathic towards the pain and distress of others than males (Christov-Moore et al., 2014). Females also display greater concern and sympathetic behavior towards others (Eisenberg and Lennon 1993; Mesch et al. 2011) starting from a young age. Females are more averse to harming others in moral dilemmas, even in cases where harming another may save lives. This is referred to as an increased disposition towards deontological (avoiding harm) over utilitarian (maximize outcomes) decisions (Friesdorf et al. 2015). This sex disparity in empathy for the pain of others may be due to a more prominent evolutionary role in nurturing behavior (Christov-Moore et al. 2014). Furthermore, recent studies in our lab and others have shown that prosocial behavior and deontological moral reasoning are predicted by somatomotor resonance with others’ pain (Christov-Moore and Iacoboni 2016a; Christov-Moore et al. 2017; Morelli et al. 2014).
Taken together, these findings may imply that females greater concern for others may partly stem from greater somatomotor resonance: indeed, females consistently score higher on measures of trait empathy that rate one’s empathic responses to others’ distress (Christov-Moore et al. 2014). Females show greater vicarious responses to the sight or knowledge of another person in pain or distress (Christov-Moore et al. 2014; Yang et al. 2009), and event-related potential (ERP) studies have found that females show greater amplitudes in somatosensory processing-related ERP waveforms in response to humans’ suffering (Groen et al. 2013). This increased resonance generalizes beyond responses to pain. Indeed, MEG and EEG demonstrate oscillatory activity in the 10–20 Hz range at rest in somatomotor regions that desynchronizes during both action performance and observation (the mu rhythm)(Muthukumaraswamy and Johnson 2004). Two studies have reported increased mu suppression in female subjects (Cheng et al. 2008; Yang et al. 2009). Females also exhibit greater facial mimicry when viewing emotional facial expressions (Sonnby-Borgström 2002).
If resonance is important for empathy but requires a continuous interplay with mechanisms of control, sex differences in empathy may be due to differences in resonance, its control, or both. Here we use brain imaging in a large subject cohort to attempt to resolve this issue. We studied with functional MRI 70 healthy subjects (36 females) while they observed a hand receiving painful (Pain condition) and non-painful (Touch condition, as a control) stimuli (Avenanti et al. 2009) and later self-reported their own trait empathy (the Interpersonal Reactivity Index or IRI)(Davis 1983). We examined sex differences in both brain activity and connectivity, using a permutation-based non-parametric approach (Winkler et al. 2014), which has been shown to have better validity than parametric methods for clusterwise inference (Eklund et al. 2016). Indeed, assuming continuous interactions between resonance and control implies that greater resonance may result not only from ‘primary’ greater activity in resonance but also from reduced connectivity with control systems, so that control mechanisms are less effective on resonance activity. This experiment aims to resolve this ambiguity.
Methods
Subjects
Subjects were 70 ethnically diverse adults aged 18–35 (36 female, 34 male). All subjects were recruited from the local community through fliers. Eligibility criteria included: right handed, no prior or concurrent diagnosis of any neurological (e.g., epilepsy, Tourette’s syndrome), psychiatric (e.g., schizophrenia), or developmental (e.g., ADHD; dyslexia) disorders, no history of drug or alcohol abuse, all verified via phone screening. No neuropsychological measures were collected. We did not collect data on educational level or IQ. These subjects’ task-based and structural imaging data have been included in methods and analyses reported in previous papers (Christov-Moore and Iacoboni 2016a, 2016b; Christov-Moore et al. 2017). These prior studies examined correlations between Needle Test activation with an external measure of prosociality in one sample (n=20); examined effects of neuromodulation on prosociality (n=58); and examined correlations between Needle Test activation with moral decision-making (n=19), respectively. None of these, however, examined sex differences in activation as is being done here.
Trait Empathy Assessment
Interpersonal Reactivity Index (IRI):
The IRI (Davis 1983) is a widely used (Avenanti et al. 2009; Pfeifer et al. 2008) questionnaire designed to measure both “cognitive” and “emotional” components of empathy. It consists of 24 statements that the subject rates on a 5-point scale ranging from 0 (Does not describe me very well) to 5 (Describes me very well). The statements are calculated to test four theorized subdimensions of empathy:
Fantasizing Scale (FS): the tendency to take the perspective of fictional characters.
Empathic Concern (EC): sympathetic reactions to the distress of others.
Perspective Taking (PT): the tendency to take other’s perspective
Personal Distress (PD): aversive reactions to the distress of others
Subjects filled out the IRI at the end of each experimental session in a closed room, unobserved. The IRI was applied at this time point (following the scans) in order to ensure that the questionnaire did not prime empathic processes during scanning. While we cannot exclude that the stimuli shown in the scanning sessions may have influenced the IRI scores, this is a less likely possibility, since IRI is considered a fairly stable measure of self-reported dispositional empathy: no studies to our knowledge have found an effect of intervention on IRI scores, and a large (103 males and 102 females) study contrasting children and adolescents found that IRI subscale scores changed slightly throughout adolescence in accordance with well-documented developmental changes (increases in empathic concern and perspective-taking, and decreases in personal distress – Davis & Franzoi, 1991). Their scores were summed for each subdimension (measured by 6 items) to make 4 scores per subject. Cronbach’s alpha, a measure of reliability, was assessed for the IRI using SPSS (FS=0.752, EC=0.792, PT=0.816, PD=0.839).
Functional MRI Tasks
Needle Test (NT):
The stimuli were 20 full-color videos previously used by Bufalari et al. (2007) and used with permission by their research group, depicting an apparently male human hand being pierced by a hypodermic syringe (Pain condition) and touched by a wooden q-tip (Touch condition) in varying locations, as well as a static hand without stimulation (Hand condition) for use as a control. The run consisted of 12 trial blocks lasting 26s each, plus 8 alternating rest blocks that lasted either 5s or 10s. Each trial block consisted of 4 videos of a single condition (Pain, Touch, Hand), approximately 5s in duration each, with an interstimulus interval of 400ms. Subjects were simply instructed to watch the video clips. They were assured that the hand in the video clip was a human hand and not a model, but they were not instructed to empathize with the model nor were there any audiovisual cues to indicate pain in the model.
Three different block orders were used, and controlled to ensure an approximately equal proportion of male and female subjects were exposed to each block order. All tasks were coded within Presentation (created by Neurobehavioral Systems).
MR Image Acquisition
All neuroimaging data was acquired via a series of MRI scans conducted in a Siemens Trio 3T scanner housed in either the Staglin Center for Cognitive Neuroscience (n=18) or the adjacent Ahmanson-Lovelace Brain Mapping Center (n=52) at UCLA, for a total of 70 imaging datasets (1 per subject). Gender makeup did not differ meaningfully between scanners (SCCN = 9 females/9 males | ALBMC = 27 females/25 males). Scanner was not included as a covariate in the original analysis reported here, but a follow-up analysis including scanner as covariate showed identical sex differences in activation. Functional images were collected over 36 axial slices covering the whole cerebral volume using an echo planar T2*-weighted gradient echo sequence (TR=2500 ms; TE=25 ms; flip angle=90 degrees; matrix size=64 × 64; FOV 20 cm; in-plane resolution=3 mm × 3 mm; slice thickness=3 mm/1 mm gap). Resting-state images were only collected in the 50 subjects acquired at the Brain Mapping Center. A high-resolution EPI structural volume was also acquired coplanar with the functional images (TR=2000 ms, TE=33 ms, 128 × 128 matrix size, FOV=256 cm; in-plane resolution=3 mm × 3 mm; slice thickness=3 mm/1 mm gap). Finally, a high-resolution T1-weighted volume was acquired in each subject (TR=2300 ms, TE=25 ms, TI=100 ms, flip angle=8°, matrix size=192×192, FOV=256 cm, 160 slices), with approximately 1 mm isometric voxels (1.3 × 1.3× 1.0 mm).
Functional MRI Analysis
Analyses were performed in FEAT (FMRI Expert Analysis Tool), part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). After motion correction using MCFLIRT, task-based fMRI data was temporally high-pass filtered with a cutoff period of 70 seconds, approximately equal to one rest-task-rest-task period. Resting fMRI data was high-pass filtered with a cutoff period of 100 seconds. All data was smoothed using a 6 mm Gaussian FHWM algorithm in 3 dimensions. In order to remove non-neuronal sources of coherent oscillation in the relevant frequency band (.01-.1Hz), all preprocessed data was subjected to probabilistic independent component analysis as implemented in MELODIC (Multivariate Exploratory Linear Decomposition into Independent Components) Version 3.10, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Noise components corresponding to head motion, scanner noise, cardiac/respiratory signals were identified by observing their localization, time series and spectral properties and removed using FSL’s regfilt command. Each subjects’ functional data was coregistered to standard space (MNI 152 template) via registration of an averaged functional image to the high resolution T1-weighted volume using a six degree-of-freedom linear registration and of the high-resolution T1-weighted volume to the MNI 152 template via nonlinear registration, implemented in FNIRT.
Task fMRI analysis
The BOLD response was modeled using an explanatory variable (EV) consisting of a boxcar function describing the onset and duration of each relevant experimental condition (task conditions, rest, instruction screen) convolved with a double gamma HRF to produce an expected BOLD response. The temporal derivative of each task EV was also included in the model. Functional data were then fitted to the model using FSL’s implementation of the general linear model. A second-level analysis was carried out to examine significant differences between male and female subjects. Resultant images were analyzed at the group level with Randomise (Winkler et al. 2014) using 50,000 permutations of group affiliation (male or female) and automatic outlier deweighting and then cluster-corrected for multiple comparisons using Gaussian Random Field theory at a z-threshold of 2.3 and p-value cutoff of .05.
Resting and Dynamic Connectivity
To test whether functional interactions between somatomotor areas and top-down control regions exist and are correlated with sex, we performed a test of generalized psychophysiological interactions (gPPI)(Mclaren et al. 2012) and resting state functional connectivity on regions of interest (ROI’s) where significant sex differences in activation were observed (three ROI’s in somatomotor cortex) as well as three areas of a priori relevance for pain observation that were activated by the task: left Dorsolateral Prefrontal Cortex (DLPFC), left Anterior Insula (AI) and Anterior Cingulate Cortex (ACC).
To create the ROI’s, time series were extracted from 6mm diameter seeds created in standard (MNI 152) space centered on the voxels showing highest differences in activation between males and females: left Hand S1 (x=−38mm, y=−26mm, z=58mm), left superior M1 (x=−10mm, y=−16mm, z=72mm) and right superior S1 (x=14mm, y=−36mm, z=74mm) respectively. Time series were similarly extracted from left DLPFC (x=−44mm, y=28mm, z=32mm), left AI (x=−30mm, y=20mm, z=−2mm) and ACC (x=0mm, y=6mm, z=30mm), using peaks of activation for the task.
To assess dynamic, task-related changes in connectivity, we modeled activity using the following EV’s: Psychological, consisting of a boxcar functions modeling the onset and duration of each task condition, convolved with a canonical double-gamma HRF; physiological, consisting of the ROI’s time series (one per analysis); and a generalized psychophysiological interaction (gPPI) for each task condition (Pain, Touch/Control or Hand), modeling the interaction between the expected BOLD response to each condition and the time series of interest. These separate gPPI estimates were then contrasted at the group level in a second-level analysis. This allowed us to test for voxels that display significant changes in correlation with the time series of the ROI for the task contrast of interest (Pain>Touch).
To assess resting connectivity, a single EV was created for each ROI using the average time series from that ROI (extracted using FSL’s fslmeants command). We regressed this time series against the whole brain.
First level analyses were carried out using FSL’s implementation of the general linear model (FEAT). Second-level analyses were carried out to examine sex differences in gPPI and resting connectivity parameter estimates, with Randomise (Winkler et al. 2014) using 50,000 permutations of group label and automatic outlier deweighting. Resultant images were cluster corrected at a z-threshold of 2.3 and p-value cutoff of .05.
Results
IRI
To assess sex differences in self reported empathy, we used repeated-measures ANOVA with sex as between-subjects effect and empathy as repeated measure. We found an effect of sex on empathy (F=12.586, p=.001, η2=.099). After performing tests of normality for scores on each subscale, we performed corresponding two-tailed parametric (Unpaired t-tests, PD and FS subscales) and non-parametric (Mann-Whitney U tests, EC and PT subscales) analyses comparing individual subscales scores between sexes (table 1).
Table 1.
Means (with 95% confidence intervals) and standard deviations for each IRI subscale by sex. Females scored higher on FS (fantasizing), EC (empathic concern) and personal distress (PD), but not on PT (perspective-taking).
| Means and Standard Deviations for each IRI subscale by sex | ||||
|---|---|---|---|---|
| FS | 18.95(17.32,20.58) | 4.96 | 21.55(19.98,23.12) | 4.9 |
| EC | 22.26(20.85,23.67) | 4.29 | 24.2(22.65,25.74) | 4.82 |
| PT | 21.08(19.5,22.66) | 4.8 | 20.42(18.82,22.02) | 5.01 |
| PD | 11.45(9.69,13.21) | 5.35 | 15.3(13.56,17.04) | 5.45 |
Females scored higher in Empathic Concern (Z=2.065, p=.039, d=.425), Personal Distress (t=3.148, p=.002, d=.713) and Fantasizing (t=2.330, p=.022, d=.527) There was no significant sex difference on Perspective-Taking (Z=1.129, p=.259, d=.134).
We also looked at correlations between the Needle>Touch parameter estimates for the three main loci of sex differences (left hand S1, right superior S1, left M1) and the IRI subscales across the total sample and in the male and female subjects alone. The correlations across all subjects were nonsignificant. However, in the female sample alone (but not the male sample), there were significant correlations between the Perspective-Taking subscale and the Needle>Touch parameter estimate for the right superior S1 (R=.361, p=.031) and the left hand area of S1 (R=.358, p=.032), though these did not survive correction for multiple comparisons.
Brain Imaging
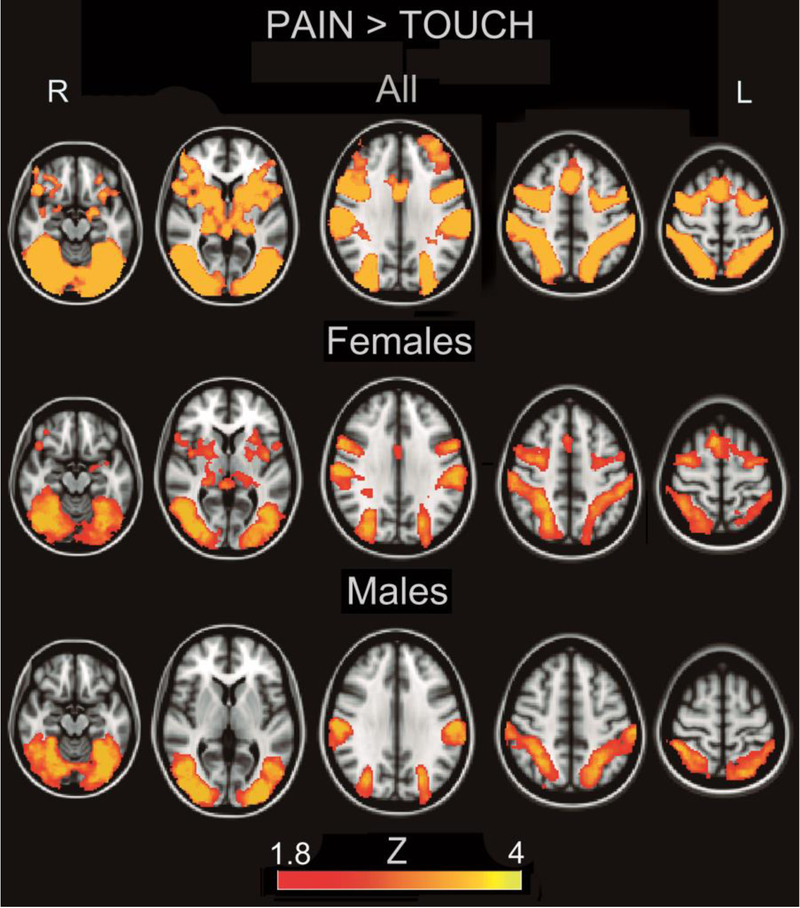
We contrasted activation while subjects were observing a hand receiving a painful stimulation from a hypodermic syringe, with non-painful tactile stimulation from a q-tip, in order to isolate variance pertinent to the vicarious processing of pain. Consistent with prior studies on empathy for pain (Lamm et al. 2011), subjects activated two broad clusters (cluster 1: 50188 voxels, p=.00052, cluster 2: 34328 voxels, p=.00146) of areas including bilateral amygdala, thalamus and insula, as well as lateral orbitofrontal, dorsolateral prefrontal cortex, premotor/motor cortex, somatosensory cortex and dorsal anterior cingulate (figure 1, supplementary table 1). When analyzed separately (figure 1 and table 2), males activated a cluster of areas encompassing visual cortex and parietal areas (40043 voxels, p=.0062), while females activated a cluster that additionally recruited dorsolateral prefrontal cortex, dorsal anterior cingulate, motor cortex, premotor cortex, insula, thalamus and left amygdala (55869 voxels, p=.0034).
Figure 1.
Activation for the Contrast Pain>Touch Overall and By Sex. Heat maps correspond to Z-scores.
Table 2.
Local maxima of activation in males and females for the contrast Needle>Touch. Standard Space (MNI) Coordinates are given of local maxima.
| Males | ||||
|---|---|---|---|---|
| Z | ||||
| x | y | z | ||
| L Cerebellum | −34 | −76 | −24 | 4.11 |
| R Cerebellum | 6 | −78 | −20 | 3.94 |
| L Occipital Fusiform Cortex | −34 | −66 | −12 | 4.11 |
| R Occipital Fusiform Cortex | 36 | −66 | −8 | 4.11 |
| L Occipital Pole | −38 | −88 | 18 | 4.11 |
| R Occipital Pole | 34 | −100 | −4 | 3.85 |
| L Postcentral Gyrus | −62 | −22 | 28 | 4.11 |
| R Postcentral Gyrus | 60 | −22 | 28 | 4.11 |
| L Superior Parietal Lobule | −30 | −56 | 54 | 3.63 |
| R Superior Parietal Lobule | 38 | −46 | 54 | 3.94 |
| L Supramarginal Gyrus | −58 | −24 | 28 | 4.11 |
| R Supramarginal Gyrus | 60 | −22 | 28 | 4.11 |
| Females | ||||
| Z | ||||
| x | y | z | ||
| Anterior Cingulate | 0 | −2 | 28 | 2.21 |
| L Amygdala | −22 | −4 | −16 | 2.11 |
| L Anterior Insula | −30 | 16 | 6 | 2.7 |
| L Inferior Frontal Gyrus, Pars opercularis | −52 | 8 | 16 | 3.94 |
| R Inferior Frontal Gyrus, Pars opercularis | 56 | 10 | 16 | 3.42 |
| L Insula | −42 | −2 | 2 | 3.57 |
| R Insula | 42 | 0 | 2 | 2.72 |
| L Lateral Occipital Cortex | −48 | −74 | 4 | 4.11 |
| R Lateral Occipital Cortex | 44 | −78 | 4 | 4.11 |
| L Occipital Pole | −30 | −90 | 18 | 3.85 |
| R Occipital Pole | 38 | −88 | 4 | 4.11 |
| L Postcentral Gyrus | −60 | −20 | 28 | 3.95 |
| R Postcentral Gyrus | 60 | −20 | 32 | 4.11 |
| L Precentral Gyrus | −54 | 8 | 28 | 4.11 |
| R Precentral Gyrus | 56 | 8 | 32 | 3.86 |
| R Precentral Gyrus | 34 | −6 | 58 | 3.63 |
| L Putamen | −24 | −2 | 6 | 2.64 |
| R Putamen | 22 | 0 | 6 | 2.99 |
| R Superior Frontal Gyrus | 6 | 14 | 62 | 3.85 |
| L Supramarginal Gyrus | −60 | −26 | 36 | 4.11 |
| R Supramarginal Gyrus | 58 | −22 | 38 | 4.11 |
| L Thalamus | −14 | −26 | 12 | 2.51 |
| R Thalamus | 20 | −28 | 0 | 3.19 |
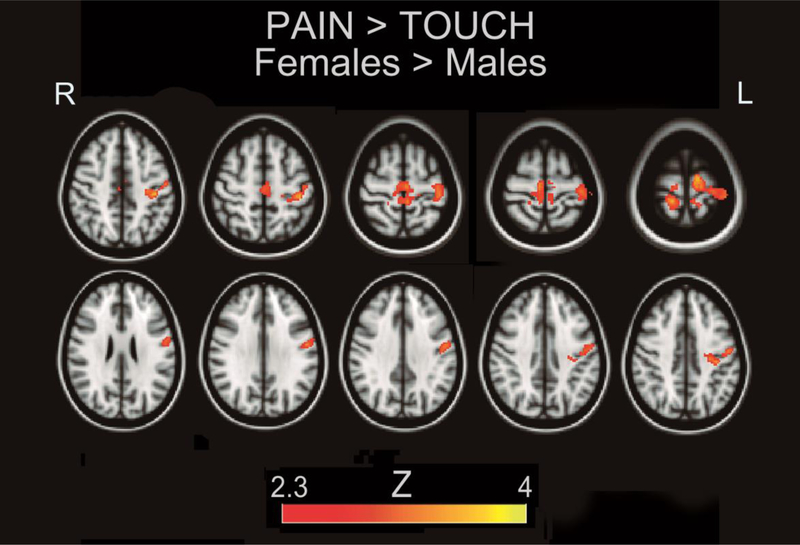
Upon comparison, the contrast Needle>Touch × Female>Males showed significantly increased activity in a cluster encompassing left M1 and bilateral S1 (figure 2, table 3). The contrast Needle>Touch × Males>Females did not show any areas of significantly higher signal for males.
Figure 2.
Females show relatively higher somatomotor activation for the contrast Pain>Touch. Heat maps correspond to Z-scores.
Table 3.
Local maxima of greater activation in females for the contrast Pain>Touch. Standard Space (MNI) Coordinates are given of local maxima.
| Z | |||||
|---|---|---|---|---|---|
| R Postcentral Gyrus | 14 | −36 | 74 | 3.38 | |
| L Postcentral Gyrus | −40 | −28 | 60 | 3.84 | |
| L Postcentral Gyrus | −36 | −26 | 46 | 3.94 | |
| L Precentral Gyrus | −10 | −16 | 72 | 3.57 | |
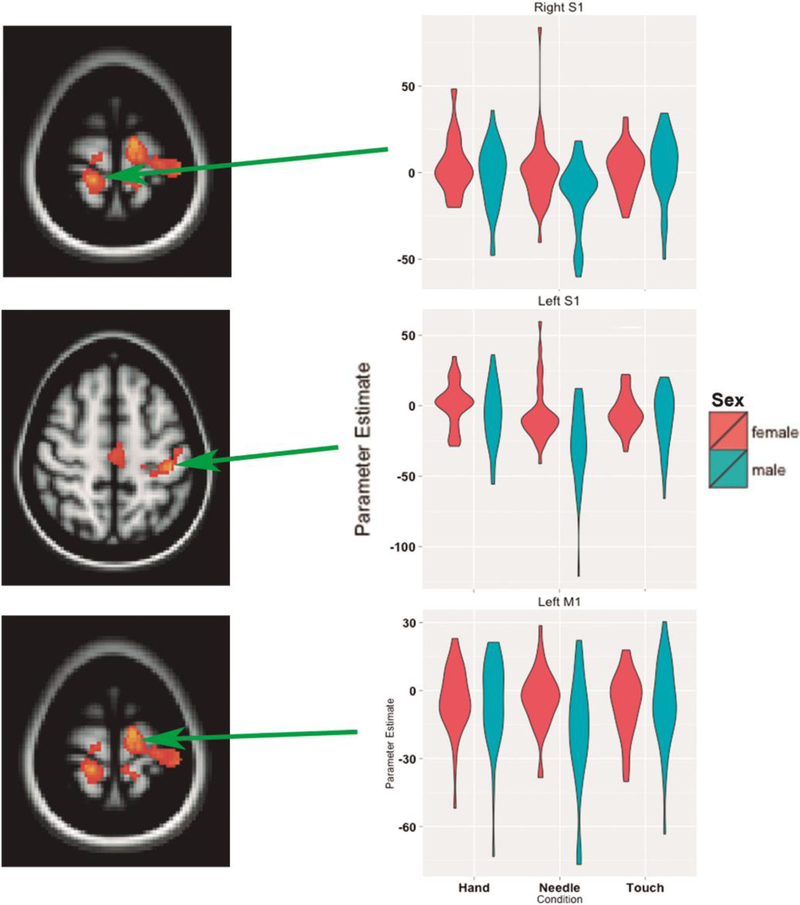
We additionally performed a post-hoc examination of parameter estimates for each condition in the area of interest, and found that the Needle>Touch sex differences have the origin in higher signal reduction in the male subjects for the Needle vs. Touch condition (Figure 3).
Figure 3.
Sex differences for the contrast Pain>Touch result from differences in deactivation. Individual parameter estimates from selected peaks are displayed in violin plots.
In order to ascertain whether these results were due to differences in somatomotor resonance or interactions between resonance and putative control areas, we examined the functional connectivity of three ROI’s highlighted by the activation contrast (left M1, left S1 and right S1) and a priori ROI’s (left DLPFC, left AI) using a generalized psychophysiological interaction analysis (gPPI)(Mclaren et al. 2012). Broadly speaking, we sought to examine whether the observed differences in activation were due to the influence of other regions. We did not find evidence of sex differences in dynamic changes in connectivity for the task contrast of interest in any of these seed regions.
Last we sought to examine whether males showed evidence for greater resting connectivity between putative highlighted control areas and somatomotor areas implicated here in sex differences. We used the same six seed ROI’s used in the gPPI analyses. These analyses failed to find significant connectivity differences between males and females.
Discussion
When observing a human hand receiving painful stimuli, females demonstrated higher activity in central, primary somatomotor areas relative to males, consistent with previous studies revealing sex differences in mu suppression in central somatomotor areas (Cheng et al. 2008; Yang et al. 2009). These findings do seem to support the notion that females have increased bottom up resonance with the pain of others. However, the picture is complicated by the parameter estimates in each condition underlying the contrast. Males showed reduced signal in response to the needle condition than female subjects. This could perhaps be interpreted as greater suppression of resonance in the male subjects. However, the lack of significant connectivity differences in this study seem to rule out that the effects we are seeing here are due to reduced efficacy of top down control mechanisms on bottom up resonance in females. We think, however, that in interpreting these findings, it may be better to take a more nuanced stance.
It should be noted that the stimuli we used in this study have been used in Transcranial Magnetic Stimulation (TMS) studies of motor excitability, demonstrating a modulatory effect on motor cortex excitability by the perception of seeing someone else in pain (Avenanti et al. 2005). Motor cortex excitability is a pre-reflective parameter that has been shown repeatedly to track stimulus properties in a bottom up fashion (Aziz-Zadeh et al. 2004; Fadiga et al 1996; Gangitano et al. 2001). The anatomical location of the sex differences we observed in BOLD fMRI fits well the TMS motor excitability studies, thus reinforcing the notion that what we are seeing here is indeed a sex difference in bottom up processing.
However, even ‘motor resonance’ (as often the stimulus-driven modulation of motor excitability is called when the stimulus involves actions) can be modulated by top down control mechanisms (Cross et al. 2013). Recent studies all point to continuous interactions between bottom up somatomotor representations of harm to others and top down control mechanisms supporting deliberate prosocial decision making. Indeed, we have recently reported that: 1) brain activity while watching pain inflicted to others predicts generosity in a costly altruism game (Christov-Moore and Iacoboni 2016a); 2) this predictive relationship is causal, not simply correlational because brain perturbation TMS studies targeting prefrontal areas inversely predicting costly altruism increase generosity (Christov-Moore et al 2016b); 3) brain activity while watching pain inflicted to others also predicts deontological decisions in hypothetical moral dilemmas (Christov-Moore et al. 2017).
The tight relationships between the pre-reflective responses to seeing someone in pain (a bottom up process) and deliberate prosocial decision making (a top down process) these studies suggest, make it difficult to conceive strong individual differences (sex differences, in this case) in one processing stream (bottom up, in this case) and no differences whatsoever in the other processing stream (top down). Hence, while this study may suggest that the most prominent sex differences in empathy for pain are related to relatively greater bottom up somatomotor representations of harm to others in females, sex differences due to interactions between bottom up and top down mechanisms of empathy may require further investigations and perhaps a more sophisticated theoretical framework than the bottom-up/top-down dichotomous framework
In considering potential other factors driving these findings, it is pertinent to consider effects of similarity with the observed hand, which is seemingly male and light-skinned. However, two facts seem to go against this direction: First, regarding the hand’s color, the male and female groups had similarly low proportions of dark-skinned (Indian and Black) subjects (5/37 females and 3/35 males). Second, if what we are observing were simply a difference in resonance for a similar vs. dissimilarly gendered target, we would expect an opposite effect, i.e. greater resonance in the male subjects.
Furthermore, effects along this interpretation presuppose contextual modulation – most likely top-down in nature, which again is unsupported by our connectivity analyses.
However, this may be due to the conservative nature of permutation-based analyses. Or it may reflect more complex mechanisms of control than we are able to pick up with this design and/or technology (fMRI). While we provide connectivity-based null results supporting sensorimotor resonance as a locus of sex differences in empathy for pain, these are exclusively based on correlations between single functionally-defined seeds and the rest of the brain (as opposed to between multiple a priori seeds spanning the brain). Elucidating this issue may require more multivariate approaches. Ongoing studies on this subject in our group are incorporating whole-brain network-based unsupervised analyses of resting-state data to elucidate the sex differences in activation and connectivity we have observed here as well as their relation to empathy (Christov-Moore et al., In Preparation). Future studies should apply similar approaches to task data like that reported here.
We cannot exclude the possibility that the scanner task may have had an effect on the IRI assessment, as this was collected at the end of the experimental session. However, we concluded it a far more problematic possibility that administering the IRI prior to the scanning might differently prime subjects to empathize during the task, creating a difficult-to-model interaction effect. Indeed, there is evidence that IRI subscale scores change throughout adolescence in accordance with well-documented developmental changes (increases in empathic concern and perspective-taking, and decreases in personal distress – Davis & Franzoi, 1991). However, no studies to our knowledge have found a significant effect of task exposure on IRI scores.
To conclude, we propose here that observed sex differences in empathy for pain could be due to at least two mechanisms: sex differences in bottom-up resonance with someone else’s pain, (or emotion), or in controlling resonance via interactions between control and resonance processes. This study supports the former as the prevalent process, though we cannot conclusively rule out other possible mechanisms suppressing somatomotor resonance in males. We also emphasize that recent studies suggesting continuous interactions between bottom-up and top-down processes make it problematic to claim differences in one processing stream but not the other, and that perhaps we do need a more nuanced theoretical framework to interpret these interactions.
Current directives within the NIH (http://grants.nih.gov/reproducibility/index.htm) and recent analyses of neuroscience research (McCarthy et al., 2012) emphasize the necessity of a) higher sample sizes (NIH directive NOT-OD-15–103), b) attention to sex differences in order to aid diagnosis and treatment of psychiatric disorder (NIH directive NOT-OD-15–102) and c) permutation-based high-level statistics (Eklund et al. 2016). This study conforms to these directives and concerns, and suggests that neuroimaging analyses of empathy in healthy and clinical populations as well as interventions aimed at empathy in clinical populations should take sex into account.
Given the foundational role of empathy in social cognition (Zaki and Ochsner 2012), these results may be a step toward building a neurobiologically based framework to better understand sex disparities in conditions with inefficient social functioning, such as schizophrenia (Hafner 2003), psychopathy (Nicholls and Petrila 2005) and autism spectrum disorder (Halladay et al. 2015), as well as sex disparities in empathic responses, prosocial behavior (Christov-Moore et al. 2014) and moral reasoning (Friesdorf et al. 2015). This will hopefully aid in the greater goal of incorporating sex differences into the study of normal and pathological brain function.
Supplementary Material
Funding
This work was supported by the National Institute of Mental Health under grant R21 MH097178 to M.I., and by the National Science Foundation under a Graduate Fellowship Grant DGE-1144087 to L.C.M. For generous support the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund.
Footnotes
Compliance with Ethical Standards
The authors have no conflicts of interest to declare. All recruitment and experimental procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- Avenanti A, Bueti D, Galati G, and Aglioti SM (2005). Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci, 8, 955–960. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio-Paluello I, Bufalari I, and Aglioti SM (2009). The pain of a model in the personality of an onlooker: Influence of state-reactivity and personality traits on embodied empathy for pain. NeuroImage, 44(1), 275–283. 10.1016/j.neuroimage.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J (2004) Left hemisphere motor facilitation in response to manual action sounds. Eur J Neurosci 19(9):2609–2612. [DOI] [PubMed] [Google Scholar]
- Betti V, and Aglioti SM (2016). Dynamic construction of the neural networks underpinning empathy for pain. Neurosci Biobehav R, 63, 191–206. 10.1016/j.neubiorev.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, … Fierro B (2011). Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain, 12(2), 185–191. 10.1007/s10194-011-0322-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalari I, and Ionta S (2013). The social and personality neuroscience of empathy for pain and touch. Front Human Neurosci, 7(July), 393 10.3389/fnhum.2013.00393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Lee PL, Yang CY, Lin CP, Hung D, and Decety J (2008). Gender differences in the mu rhythm of the human mirror-neuron system. PLoS ONE, 3(5), 1–7. 10.1371/journal.pone.0002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L, and Iacoboni M (2016a). Self-other resonance, its control and prosocial inclinations: Brain-behavior relationships. Hum Brain Mapp, 37(4), 1544–1558. 10.1002/hbm.23119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L, Sugiyama T, Grigaityte K, Iacoboni M (2016b) Increasing generosity by disrupting prefrontal cortex. Soc Neurosci 919(June):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-Moore L, Simpson EA, Coude G, Grigaityte K, Iacoboni M, and Ferrari PF (2014). Empathy: Gender effects in brain and behavior. Neurosci Biobeh R, 46(P4), 604–627. 10.1016/j.neubiorev.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov-moore L, Conway P, Iacoboni M (2017) Deontological Dilemma Response Tendencies and Sensorimotor Representations of Harm to Others. Front Integr Neurosci 11(December):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross KA, Torrisi S, Reynolds Losin EA, Iacoboni M (2013) Controlling automatic imitative tendencies: interactions between mirror neuron and cognitive control systems. Neuroimage 83(310):493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, and Iacoboni M (2005). Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neuro, 9(1), 28–30. 10.1038/nn1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH (1983). Measuring Individual Differences in Empathy: Evidence for a Multidimensional Approach. J Pers Soc Psy, 44(1), 113–126. 10.1037/0022-3514.44.1.113 [DOI] [Google Scholar]
- Davis MH, Franzoi SL (1991) Stability and change in adolescent self-consciousness and empathy. J Res Pers 25(1):70–87. [Google Scholar]
- Domes G, Schulze L, Böttger M, Grossmann A, Hauenstein K, Wirtz PH, … Herpertz SC (2010). The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp, 31(5), 758–769. 10.1002/hbm.20903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, and Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, and Lennon R (1993). Sex differences in empathy and related capacities. Psychol Bull, 94(1), 100–131. 10.1037/0033-2909.94.1.100 [DOI] [Google Scholar]
- Fox PT, and Friston KJ (2012). Distributed processing; distributed functions? NeuroImage, 61(2), 407–426. 10.1016/j.neuroimage.2011.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesdorf R, Conway P, and Gawronski B (2015). Gender differences in responses to moral dilemmas: a process dissociation analysis. Pers Soc Psychol Rev 42, 696–713. doi: 10.1177/0146167215575731 Gleichgerrcht, [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy F, and Pascual-Leone A (2001). Phase-specific modulation of cortical motor output during movement observation. NeuroReport, 12, 1489–1492. [DOI] [PubMed] [Google Scholar]
- Groen Y, Wijers AA, Tucha O, and Althaus M (2013). Are there sex differences in ERPs related to processing empathy-evoking pictures? Neuropsychologia, 51(1), 142–155. 10.1016/j.neuropsychologia.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Häfner H (2003). Gender differences in schizophrenia. Psychoneuroendocrino, 28 Suppl 2: 17–54. 10.1016/S0306-4530(02)00125-7 [DOI] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, … Szatmari P (2015). Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority. Mol Autism, 6, 36 10.1186/s13229-015-0019-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Silani G, Preuschoff K, Batson CD, and Singer T (2010). Neural Responses to Ingroup and Outgroup Members’ Suffering Predict Individual Differences in Costly Helping. Neuron, 68(1), 149–160. 10.1016/j.neuron.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Iacoboni M (2009). Imitation, empathy, and mirror neurons. Annu Rev Psych, 60(0066–4308, 0066–4308), 653–670. 10.1146/annurev.psych.60.110707.163604 [DOI] [PubMed] [Google Scholar]
- Ingersoll B, and Schreibman L (2006). Teaching reciprocal imitation skills to young children with autism using a naturalistic behavioral approach: Effects on language, pretend play, and joint attention. J Autism Dev Disord, 36(4), 487–505. 10.1007/s10803-006-0089-y [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, … Habel U (2007). Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia, 45(12), 2744–2754. 10.1016/j.neuropsychologia.2007.04.012 [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, and Singer T (2011). NeuroImage Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. 10.1016/j.neuroimage.2010.10.014 [DOI] [PubMed] [Google Scholar]
- Machado S, Bittencourt J, and D. M (2008). Therapeutic applications of repetitive transcranial magnetic stimulation in clinical neurorehabilitation. Func Neurol, 23(3), 113–122. [PubMed] [Google Scholar]
- Masten CL, Morelli SA, and Eisenberger NI (2011). An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. NeuroImage, 55(1), 381–388. 10.1016/j.neuroimage.2010.11.060 [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ (2012) Sex differences in the brain: The not so inconvenient truth. J Neurosci 32(7):2241–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaren DG, Ries ML, Xu G, and Johnson SC (2012). NeuroImage A generalized form of context-dependent psychophysiological interactions ( gPPI ): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesch DJ, Brown MS, Moore ZI, and Hayat AD (2011). gender differences in charitable giving. Intll J Nonprofit Vol Sect Market, 21(July), 3–12. 10.1002/nvsm [DOI] [Google Scholar]
- Miller EK, and Cohen JD (2001). An Integrative Theory of Prefrontal Cortex Function. Ann Rev Neurosci, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Morelli SA, Rameson LT, and Lieberman MD (2014). The neural components of empathy : Predicting daily prosocial behavior. Soc Cog Affect Neurosci, 9, 39–47. 10.1093/scan/nss088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, and Johnson BW (2004). Changes in rolandic mu rhythm during observation of a precision grip. Psychophysiology, 41(1), 152–156. 10.1046/j.1469-8986.2003.00129.x [DOI] [PubMed] [Google Scholar]
- Nicholls TL, and Petrila J (2005). Gender and psychopathy: An overview of important issues and introduction to the special issue. Behav Sci Law, 23(6), 729–741. 10.1002/bsl.677 [DOI] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-leone A, and Wirecki TS (2016). Brain Stimulation The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul, 9(3), 336–346. 10.1016/j.brs.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Iacoboni M, Mazziotta JC, and Dapretto M (2008). Mirroring others’ emotions relates to empathy and interpersonal competence in children. NeuroImage, 39(4), 2076–2085. 10.1016/j.neuroimage.2007.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Riva F, and Zani A (2010). When neurons do not mirror the agent’s intentions: Sex differences in neural coding of goal-directed actions. Neuropsychologia, 48(5), 1454–1463. 10.1016/j.neuropsychologia.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ and Frith C (2004). Empathy for Pain Involves the Affective but not Sensory Components of Pain. Science, 303(5661), 1157–1162. 10.1126/science.1093535 [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, and Frith CD (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439(7075), 466–469. 10.1038/nature04271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF (2009). Correspondence of the brain’s functional architecture during activation and rest. Proc Nat Acad Sci U S A, 106(31), 13040–5. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnby-Borgström M (2002). Automatic mimicry reactions as related to differences in emotional empathy. Scand J Psychol, 43(5), 433–443. 10.1111/1467-9450.00312 [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, and Fernández G (2010). Testosterone reduces amygdala-orbitofrontal cortex coupling. Psychoneuroendocrino, 35(1), 105–113. 10.1016/j.psyneuen.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Volman I, Katinka Louise von Borries A, Hendrik Bulten B, Jan Verkes R, Toni I, and Roelofs K (2016). Testosterone modulates altered prefrontal control of emotional actions in psychopathic offenders. Eneuro, 3(February), 1–12. 10.1523/ENEURO.0107-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Decety J, Lee S, Chen C, and Cheng Y (2009). Gender differences in the mu rhythm during empathy for pain: An electroencephalographic study. Brain Res, 1251, 176–184. 10.1016/j.brainres.2008.11.062 [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, and Winkler P (1997). Localization of the motor hand area to a knob on the precentral gyrus A new landmark, Brain, 120, 141–157. [DOI] [PubMed] [Google Scholar]
- Zaki J, and Ochsner KN (2012). The neuroscience of empathy : progress , pitfalls and promise, Nat Neuro 15(5). 10.1038/nn.3085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.