Abstract
Cancer is an abnormal cell growth which tends to proliferate in an uncontrolled way and, in some cases, leads to metastasis. If cancer is left untreated, it can immediately cause death. The use of magnetic nanoparticles (MNPs) as a drug delivery system will enable drugs to target tissues and cell types precisely. This study describes usual strategies and consideration for the synthesis of MNPs and incorporates payload drug on MNPs. They have advantages such as visual targeting and delivering which will be discussed in this review. In addition, we considered body magnetic field to make drug delivery process more effective and safer by the application of MNPs and tumor-on-chip.
Keywords: Body magnetic fields, Cancer, Drug delivery, Magnetic nanoparticles
Introduction
The development of magnetic nanoparticles (MNPs) is promising for numerous applications. The core-shell nanoparticle is mostly designed for targeted and controlled drug delivery.1,2 MNPs can be manipulated through using magnetic fields,3,4 and such procedure is categorized as a magnetic resonance imaging (MRI).5,6 These particles have relatively low magnetization which has limited their separation in external magnetic field, even if such separation is very important, especially in case of immobilized enzymes collecting.7,8 Besides, magnetic field can be changed to achieve thermal treatment.9,10 The shell of MNPs is coated with a natural, and synthetic polymer or material copolymer.1,11,12 Researchers improve various factors such as temperature, pH, and dual stimuli responsive polymeric nanomaterials such as micelles, vesicles, gels to contribute to cancer treatment.13,14 Due to recent researches, silica nanoparticles,15,16 iron oxide MNPs, and multi-metallic MNPs, incorporated into dual stimuli responsive polymeric functionalities, have attracted attentions to biomedical applications.17-23
Today, anti-cancer drug discovery has mostly been conducted using in-vitro and animal models. Animal models for cancer such as mouse and rat models may not attribute to humans accurately, and in-vitro models may not be able to simulate tumor microenvironment (TME); thus, they are not appropriate to explore the interactions of cells and organs.24-27
Technology of tumor-on-a-chip has recently been introduced as a new approach for cancer research while providing a novel tool which considers microfabrication, biomaterials research, microfluidics, and tissue engineering all together. A tumor-on-a-chip comprises of a microchannel surrounded by a kind hydrogel matrix that is made up of collagen. This system can be used to simulate key aspects of nanoparticle transport such as nanoparticle uptake, diffusion within the extracellular matrix, and extravasation in the tumor.28-31
Currently, a number of methods have been put forth for the separation of particles such as acoustic separation, fluorescence, size-based separation, dielectrophoresis, and magnetic control, on tumor-on-a-chip systems. Among these methods, those which are based on magnetic-activated separation are more attractive since they utilize functionalized magnetic particles to capture specific targets through binding and to separate the complex by magnetic manipulation afterwards.30,32-37
In addition, to cancer diagnosis and treatment, MNPs can also be used to treat infectious diseases. Yung et al has created an organ-on-chip for blood cleansing using magnetic opsonins targeting Candida albicans. The microorganism is, then, cleaned by a micromagnetic separator.38
This review describes strategies and considerations of MNPs preparation for drug carrier applications. MNPs have some advantages such as effective targeting and delivering which will be discussed in this review. In addition, we considered body magnetic field that can further drug delivery process more effectively and safely by using MNPs.
Magnetic field of body organs
Approximately, one Hertz (Hz) frequency is known as a static magnetic field that has different effects on the body. A magnetic field with 0.8T, 22 ms and 1 Hz frequency hampers the growth of S-180 sarcoma in mice. A magnetic field with same properties has been used for patient treatments in their middle and late-stage.39 Density and electric field in the tissue and body are expressed in A/m2 or mA/m2 and V/m or mV/m. During the last years, electric or magnetic fields and current density of human body have been modeled by inducing electric fields at 50 or 60 Hz.40,41 Currently, heterogeneous models have been developed with MRI for human body with proper tissue types.42,43 Typically, cubic cells (Voxels) are measured for various distinct organs and tissues.44 Several organs and tissues have been computed and induced in an electric field.45,46 Gap-connected cells have been investigated by long cables model (Table 1).47
Table 1. Presents electric field induced in several organs and tissues at 60 Hz, 1 µT magnetic field oriented front-to-backa .
| Tissue/organ | Mean | 99th Percentile | Maximum | |||
| 50 Hz | 60 Hz | 50 Hz | 60 Hz | 50 Hz | 60 Hz | |
| Liver | 13/2 | 28/2 | 73/1 | |||
| Lung | 8/22 | 21 | 24/4 | 49 | 93/3 | 86 |
| Blood | 5/99 | 6/9 | 17/5 | 23 | 30/9 | 83 |
| Uterus | 3/81 | 9/44 | 17/0 | |||
| Prostate | 17 | 36 | 52 | |||
| Ovary | 2/40 | 5/30 | 7/87 | |||
| Breast | 18/1 | 31 | 51/6 | |||
aAdopted from Dawson et al.47
Magnetic field of cancer cell
Cancer is still one of the principal causes of death in the developed countries. Conventional therapies for cancer such as surgery, radiation, chemotherapy, and biological therapies have varied disadvantages. The tumor accessibility, the operating risk on vital organs, the cancer cells spreading throughout the body, and the lack of selectivity toward tumor cells are some of these disadvantages. Immunotherapy has been applied for the treatment of small tumors since its efficacy decreases in more advanced stages of cancer. Multimodal therapy has been applied to provide a better chance of survival.48
Recent investigation shows the presence of non-erythroid cell lines, stemming from cancer cells of human that show paramagneticproperties.49 The cell behavior can be affected with the use of high gradient magnetic filtration according to this property. The inter and intracellular free radicals (O3, NO, and NO2), molecules (O2) and salts (FeCl3) can be redistributed using Lorentz force and magnetic gradient.50,51
Recent studies have shown that the magnetic and mechanical forces can cause physical interactions that are able to change the cell shapes, functions, and fate change.52-54 Cell division can be limited by mechanical stress near the spheroid surface of cancer cells.55 The idea of magnetic behavior is enriched in the presence of iron ions and consequently leads to the difference in paramagnetic properties between them and healthy ones. Magnetic radial pressure can make tumor cells paramagnetic and limit tumor growth. Magnetic effects on mouse tumor cell were examined with luminescence, while the cell growth rate dose was not affected. In addition, human fibroblastoma (DMD-A) and human melanoma did not change in growth rate in two cell line culture when a magnetic field with magnetic flux density of 47 T was applied for 72 h. Magnetic fluid hyperthermia (MFH) is another controlled therapy which is non-invasive and supplements chemotherapy. This method can achieve a much higher rate of cancer-cell destruction, either in vitro or in vivo.56
Magnetic nanoparticles
The crystal structure, more precisely the orbital electron arrangement in Fe and some other materials creates ferromagnetic species. The iron atomic number is 26, and this electronic configuration has four unpaired electrons in the last orbital that display some associated characteristics and behavior toward other atoms. A single electron has a quantum number of Ms+(1/2) or Ms– (1/2). It is possible for the transformation of ferromagnetic materials to permanent magnet using a strong attraction by magnetic fields.57 The metallic nanoparticles are synthesized and modified, utilizing in biomedical science and engineering. The widespread application of them is unavoidable, specifically in biotechnology, magnetic separation, drug delivery, vehicle for gene delivery fields, and mainly in the field of diagnostic imaging; for example, MRI, computerized tomography (CT), positron emission tomography (PET), ultrasound, optical imaging and surface-enhanced Raman scattering (SERS). Iron oxide (Fe3O4), gold, and silver are different MNPs.58 Iron, cobalt, or nickel are used to synthesize metallic MNPs. Oxide of metal MNP scan be protected by the use of coating of silica or gold to prepare core-shell structure.1 The volume, shape, composition, and matrix viscosity of the magnets as well as the temperature are the factors which determine magnetic behavior. In a simple and useful model, MNPs with volume of V and saturation magnetization of Ms are considered as species with the shapes of spherical ellipsoids which have a permanent moment m¼MsV. Combination of metallic nanoparticles and MNPs are opening new aspects of application in medicine (Figure 1).59
Figure 1.
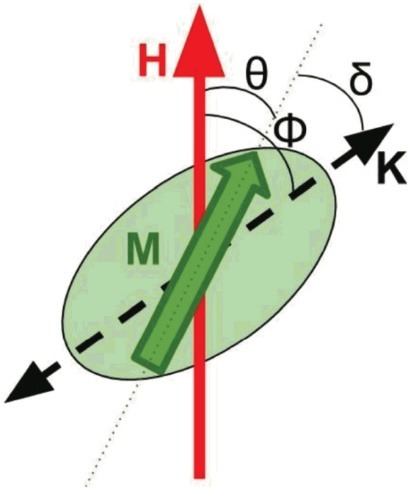
MNP model. The angle h between the magnetization M and the field H, and the angle d between M and the easy axis K, determine the energy.59 MNP: magnetic nanoparticles.
Magnetic hyperthermia nanoparticles have major potentials for clinical application. They are vehicles that are used for cell killing and damaging as well as for increasing the sensitivity to the radiation effects and/or certain anticancer drug.60,61 Tumors temperature typically fluctuates between 41–46°C (moderate hyperthermia) or >46°C (thermo ablation) for a specific duration of time.61 Tumor cells usually have higher heat sensitivity over normal cells, at milder temperatures around 43°C, resulting in damage to tumor cells only.62,63
Tumors will be warmed by using various techniques such as radio, ultrasound, or infrared waves that can stimulate fatal side effects.64-67 There is a relationship between the size distribution and the magnetic properties of the NPs. Hence, very small NPs (∼1–30 nm) do not show sufficient hyperthermia effects and too big ones (>200 nm) are not able to cross the endothelial barrier. Treatment with MNP hyperthermia was initially used in 2007 for prostate cancer. When MNPs were applied on metastatic bone tumors, they led to a reduction in the lesion and resulted in new bone formation.68
Although there are various methods to synthesize MNPs, it is necessary to develop NPs, which are chemically stable and free from oxidation by oxygen and erosion by acid or base. The MNP is coated by a shell to be protected and to remain stable in harsh chemical situations. There are many advantages in using MNPs with core/shell structure; for instance appropriate dispersion and high stability against oxidation are among them.69 There are two types of coating which are divided into organic and inorganic such as silica, carbone and precious metal, or oxides.70
The physical absorption and chemical bonding have been used for loading of different molecules on organic and inorganic bases: the tumor-recognition moieties,71,72 cell-penetrating peptides for MRI applications,73,74 as well as enzymes,75 genes,76-80 growth factors,81,82 radionucleotides,83,84 drugs,85,86 tamoxifen,87 cefradine,88 ammonium glycyrrhizinatezz,89 fludarabine,90 cisplatin and gemcitabine,91 amethopterin,88,92 mitomycin,93 paclitaxel,94,95 diclofenac sodium,96,97 and Adriamycin98 for drug delivery applications (Figures 2 and 3).99
Figure 2.
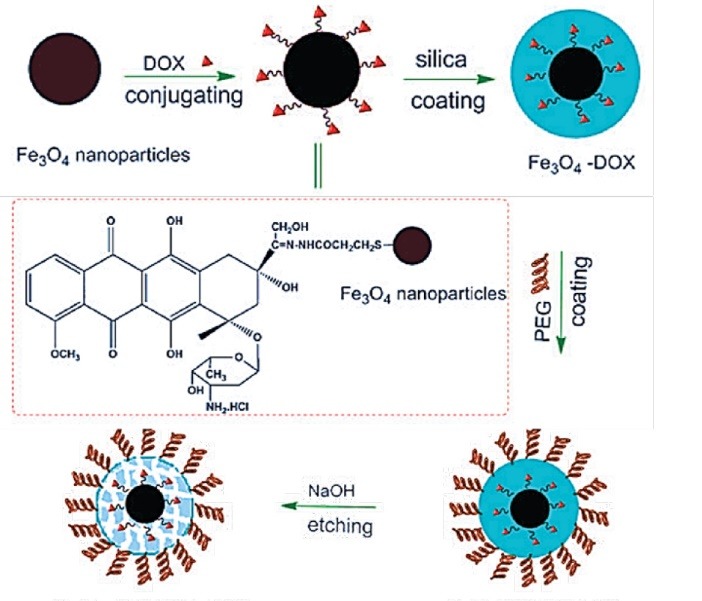
Synthetic scheme for the development of Doxorubicin loaded magnetic drug delivery system (Adopted from Mody et al99 ).
Figure 3.
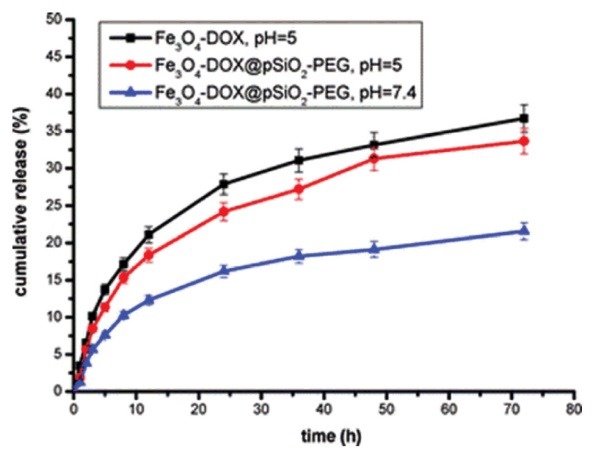
Comparative release profile of Doxorubicin in iron oxide conjugated DOX. Iron oxide conjugated DOX in silica and PEG protected shell (Adopted from Mody et al99 ). DOX: doxorubicin; PEG: polyethylene glycol.
The first polymer MNPs and micro particles were first used in the 1970s. This type of coating protects the shield from the surrounding environment and works when coupled with carboxyl groups, carbodi-imide, biotin, avidin, and other molecules. The carrier of MNPs is made up of different structural configuration of magnetic particle core such as Fe3O4 or Fe2O3.100
Function and application of MNPs
The MRI agents, based on an oral contrast type of large iron oxide particles for MRI of bowel/GI tract, were the first ones of these agents.101 Abdoscan® (Ferristene) is another oral iron-based negative MRI which has been approved in Europe. The size of these MNPs is 50 nm which have been coated with polystyrene to obtain 3.5μm particles.102 These MNPs are mainly used to take pictures from liver/spleen/lymph nodes in patients with pelvic, prostate, bladder, or breast cancer, and this process is briefly elaborated here. Relatively, iron oxide MNPs of large size are used for liver/spleen imaging such as AMI-25 (Feridex®/EndoremTM, DH = 80–200 nm) and SHU 555A (i.e., Resovist®/CliavistTM, DH = 60 nm).103,104 Iron oxide of smaller particle size is considered for lymph node/bone marrow/carotid atherosclerotic plaques imaging (AMI-227 [i.e., Sinerem®/CombidexTM, DH = 20–40 nm]).105,106
The idea for using magnetic particles as drug carriers for cancer therapy has been utilized in 1960.107 The cytotoxic activity of iron oxide MNPs is insignificant when administered at Fe concentrations up to 100 μg/mL,108 and even up to 8 mg Fe/mL in formulations such as ferumoxytol.109 The normal blood iron concentration and clinical doses of such MNPs in humans are about 33 mg Fe/kg and 0.56-8 mg Fe/kg patient body weight which is incomparable.110
In addition to afore mentioned point, MNPs potentially can play a crucial role in the new cancer treatment approaches. Among the methods of diagnosis and treatment of cancer, magnetic-activated separation is more attractive for researchers since this method utilizes functionalized magnetic particles to capture specific targets through binding and separates the complex by magnetic manipulation afterwards. The separation relies on the chemical bonds interaction and, hence, specific and selective separations of the particles are allowed.32-37 , With this approach, MNPs can also be used to treat infectious diseases. In a study, the blood cleansing from Candida albicans was done using magnetic particles.38
Drug delivery
Any agent used in drug delivery is called a drug carrier which serves to enhance the safety, selectivity, and effectiveness of drug administration. Generally, Drug carriers are used for controlled release of a therapeutic agent into circulation. Such release can be caused either by a typical diffusion of drug (slow release) or by a triggered drug deliverance to a specific target by stimuli such as pH, heat, and light. The more popular drug carriers include polymeric micelles, liposomes, microspheres, and nanoparticles.111,112
Nanoparticle carriers have considerable potential for cancer diagnosis and treatment. The most important technological benefits of NPs are high carrier capacity, incorporation of both hydrophobic and hydrophilic drugs, and high stability and feasibility of different routes of application including oral administration. The mentioned properties of NPs improve drug bioavailability and dosing frequency reduction, and in some disease may resolve the problem of nonadherence to a therapy.113-116 MNPs are a class of NPs which can be manipulated by magnetic fields.117
The nanoscience technology is a promising way to detect disease and control drug delivery.69 MNPs are very beneficial due to their unique properties of biological interaction which makes them a suitable agent for MRI. Examples of such unique proprieties of MNPs are magnetic moment, non-fouling surface in detection, diagnosis, and treatment of malignant tumors as well as cardiovascular and neurological disease.1 MNPs are carriers which are able to deliver drug in a particular area in the body. They can be mixed with drugs and injected to the specific area by an intravenous or intra-arterial injection. Reaching the site, the drug is released from the carrier due to enzymatic activity or a change in pH, osmolality, or temperature. This technique improves the efficacy of drug delivery, reduces the systemic distribution of cytotoxic drugs, and results in an efficient treatment at lower doses.118 The “tumor-on-a-chip platforms for NP-transport and testing” section will elaborate more on new MNPs implications.
Magnetic drug targeting
Magnetic drug targeting is an appropriate method for delivering drugs to diseased area which may need a minimal invasive procedure.119-121 The MNPs change the structure of the thermos-sensitive material by generating heat. In addition, the drug is released by the carrier at the tumor site and increases the effectiveness of the therapy.61 Under extensive investigation, prostate and brain tumors have been diagnosed by magnetic hyperthermia in humans.122
The ideal size of NPs for cancer therapy due to their fast tumor penetration is approximately 12 nm. It has also been shown that 50 nm nano-conjugates can be close to the optimal size for overall tumor tissue accumulation and retention compared with those smaller than 20 nm which are rapidly cleared from the bloodstream and with those larger than 200 nm which have limited tumor tissue penetration.123,124
A common occurrence in NPs is the removal of size dependence in the human vascular system. Which depends on morphological pore sizes, involved in diffusive permeability of the capillary vessels. There are some examples of morphological pore size in our body.125 The maximum size of NPs is permitting penetration through cell membranes (∼1 μm). The upper size limit for particles with rigid structures flowing in veins is 5 µm. Due to the fact that the size of typical NPs is below the narrowest capillaries, basically there is no limitation concerning delivery procedure. As it was previously mentioned, the most important limitation lies in an increase in the circulation time and the clearance from the body. Because of negative charge of the luminal surface of vascular endothelial layer, the positively charged NPs can rapidly react with endothelial cells by the formation of non-specific bonds, and a reduction in blood circulation.126 On the other hand, NPs with highly negative charges are capable of being trapped by some macrophages and organs. It is difficult for neutrally charged MNPs to be sterically stabilized. Hence, for longer blood circulation, it is necessary for MNPs to have a negative or positive surface charge for improved targeting.127
To demonstrate optimal capacity to incorporate chemotherapeutic drugs and to be effective delivery vehicles, the therapeutic MNPs should have average sizes between 10 and 100 nm with a proper coating, ζ-potential values between -15 and+15 mV, and an elongated to spherical shape. However, the preparation of such stable colloidal MNPs which are highly controllable for delivering the drugs to targets is still debatable.128
Biotransformation of MNPs
Recent investigations have expressed that the identity and properties of NPs can be changed with biological interactions.129 The molecules within the biological fluids reshape NPs’ surface and such change leads into particle aggregation enzymatic attack.130,131 NPs remodeling may be effective in regulation of transportation in physiological media, cellular internalization, and potential toxicity. The MNPs degradation may produce harmful by-products which have unexpected biological effects. On the other hand, the saturation of lysosomal compartments may occur with non-degradable NPs accumulation.132,133 A suitable external magnetic field can appropriately separate the magnetic NPs from blood as a complex biological media. Importantly, the corona varies over time depends on the stage of cell process.134 The observations show that these particles reach liver and spleen. The coating-dependent elimination of super para- magnetic iron from these organs seems to take months. Paramagnetic iron concentrates in liver and spleen and, afterwards, transforms into iron oxide phases with no magnetic properties. Particles coated by amphiphilic polymers are more persistent than polyethylene glycol of hydrophilic chains and they can be observed in vivo in liver and spleen one year after injection (Figure 4).135,136
Figure 4.
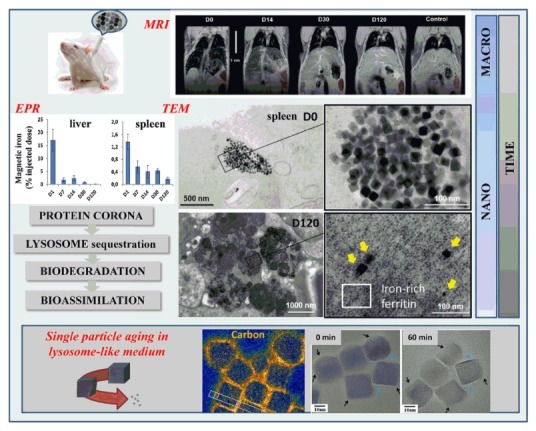
Multiscale follow-up of iron oxide nanocubes over time, using in vivo MRI in mice, ex vivo EPR quantification in organs, TEM observations of intracellular distribution and morphological biotransformations.136 MRI: magnetic resonance imaging; EPR: electron paramagnetic resonance; TEM: transmission electron microscopy.
Tumor-on-a-chip platforms for NP-transport and testing
Tumor-on-a-chip devices provide viable options for promoting the efficacy of cancer therapy.137 Today, the study, identification, and treatment of cancer tumor cells circulating (CTCs) are developed by the tumor-on-a-chip device .There are some drawbacks in conventional therapies such as low drug specificity, poor water solubility, lower therapeutic efficiency, and drug resistance which can be eliminated through the utilization of NP-based targeted drug delivery systems (Figure 5).138-140
Figure 5.
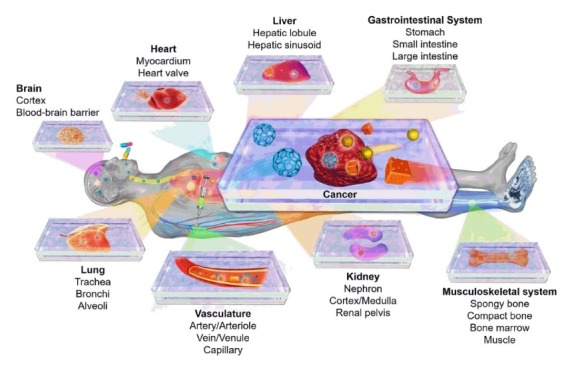
Organ-on-a-chip and disease-on-a-chip platforms for modeling human physiology and pathophysiology.140
Despite the comprehensive research in cancer biology, TME is not well known. As it was mentioned before, the disease is the leading cause of mortality in the developed countries. Hence, it seems the understanding of TME can have a pivotal role in cancer diagnosis and treatment. Technology of organs-on-chips provides effective approaches to have a deep vision on TME.128,141-143
Today, anti-cancer drug discovery has mostly been conducted using in-vitro and animal models. Cancer animal models can provide vital information about responses to anti-cancer drugs. Although the models could be low cost, the wide variations among the animals used make it difficult to obtain relevant statistics. Furthermore, animal models for cancer such as mouse and rat models may not attribute to humans accurately.144 Alongside in-vivo studies, different types of cell culture models have been used for cancer research.26,27 Such in-vitro studies may co-culture multiple cell types with patient derived cells in a matrix. Although in-vitro studies are costly and repeatable, the models may not be able to simulate TME; Therefore, they are not appropriate to explore the interaction of cells and organs.24,25
Technology of tumor-on-a-chip has recently been introduced as a new approach for cancer research while providing a novel tool which contains microfabrication, biomaterials research, microfluidics, and tissue engineering all together.28 A tumor-on-chip system is comprised of a microfluidic chip which has nutrients, waste removal functions, tissue culture, and small molecule supply. Tumor can grow on the device with a complex structure consisting of blood vessels, tumor cells, and stromal cells three-dimensionally.28-31
As it was mentioned before, a tumor-on-a-chip is comprised of a microchannel surrounded by a kind hydrogel matrix which make up of collagen. This system can be used to simulate key aspects of nanoparticle transport such as nanoparticle uptake, diffusion within the extracellular matrix, and extravasation in the tumor. This tumor platform can be explored for developing novel nanoparticles with some focus on emphasized optimizing features such as functionalization method, size, and shape. Consequently, the system can yield the most appropriate nanoparticles with characteristics which facilitate transport within the TME, resulting in more effective cancer treatments with nanoparticles.145-147
Currently, a number of methods have been put forth for the separation of particles such as acoustic separation, fluorescence, size-based separation, dielectrophoresis, and magnetic control on tumor-on-a-chip systems. Among these methods, those which are based on magnetic-activated separation are more attractive since they utilize functionalized magnetic particles to capture specific targets through binding and, thus, to separate the complex by magnetic manipulation. The separation relies on the chemical bonds interaction due to which specific and selective separation of the particles can be allowed.30,32-37
In addition to cancer diagnosis and treatment, MNPs can also be used to treat infectious diseases. Spleen plays a crucial role in filtering the circulating fluid and is important to body’s immune system. It forms immune responses against certain microorganisms such as Candida albicans. Yung et al created an organ-on-chip for blood cleansing using magnetic opsonins targeting C. albicans. The microorganism is, then, cleaned by a micromagnetic separator.38
Recent studies have attempted to clarify MNPs behavior in response to magnetic fields in 3D microfluidic models. Benhal et al have designed a sample test on-chip--and-image tracking system to assess the movement of MNPs in response to an applied magnetic field. The authors have been able to evaluate the motion of different MNPs ranging from 10 nm to 100 µm in diameter size. All of the particles seem to have displayed consistent trends: i.e., larger MNPs move faster; the particles at higher concentrations make longer needle like aggregates and move faster,; both speed and chain increase with time while yielding a final speed proportional to the square of particle diameter.148 In a similar study, Geczy et al have revealed that the resulting particles, through the encapsulation of MNPs, were monosized, highly spherical, and exhibited superparamagnetic properties. The particles’ size regime and their magnetic responses have demonstrated potentials for in-vivo intravenous applications of magnetic targeting with maximum magnetic response without blocking an organ’s capillaries.149
MNPs as a theranostic agent
Theranostic is defined as the combination of diagnostic and therapeutic agents within a single platform.150 Table 2 summarized the studies used theranostic multimodal MNPs for diagnosis and treatment of chronic disease especially cancer. The conventional methods for diagnosis and treatment of cancer are biopsy and chemotherapy -- which is very invasive and time-consuming in comparison. Besides, there are several limitations for chemotherapy to achieve desirable results. The entire tumor removal is not applicable by chemotherapy. Therefore, the satisfactory diagnosis and treatment of the metastatic tumor remain a challenge. Such challenge encourages scientists to research on NPs for the detection of and the cure for cancer by paclitaxel and Nano-formulated anthracyclines. Specific drugs and ligands have been conjugated on the NPs surfaces which interact with proliferating cells of tumor leading into accumulation at the tumor. The targeting system provides low chemo-resistance and cytotoxicity and induces the expression of apoptotic genes.151,152
Table 2. The studies that used theranostic multimodal MNPs for diagnosis and treatment of chronic diseases .
| Method of imaging | Nanoparticle | Disease | Therapeutic agent | Target | Ref. |
| Optical | PLGA | Cancer | Camptothecin | Folate receptor | 153 |
| Optical | Poly(isobutylene-alt-maleic anhydride) | Cancer | Paclitaxel | - | 154 |
| NIRF | PEG-hyaluronic acid | Cancer | Irinotecan | Hyaluronic acid | 155 |
| Raman spectroscopy | Gold | Cancer | Doxorubicin | - | 156 |
| MRI | SPION | Allograft rejection | DGKa-pDNA | CD3 antibody | 157 |
| MRI | SPIO | Immune response detection | - | - | 158 |
| Ultrasound | Hyaluronic acid encapsulated with MnO2 | Cancer | Indocyanine | Hyaluronic acid | 159 |
| Ultrasound | Mesoporous silica | Cardiac stem cell therapy | IGF | - | 160 |
| PET | T7 phage nanoparticle | Cancer | - | RGD | 161 |
| CT | Glycol-chitosan-coated gold | Cerebrovascular thrombi detection | tPA | Fibrin-binding peptide | 162 |
CD: cluster of differentiation; CT: computerized tomography; DGKa: diacylglycerol kinase alpha; IGF: insulin-like growth factor; MNPs: magnetic nanoparticles; MRI: magnetic resonance imaging; NIRF: near infrared fluorescence; PET: positron emission tomography; PEG: polyethylene glycol; PLGA: poly lactic-co-glycolic acid; RGD: arginylglycylaspartic acid; SPIO: superparamagnetic iron oxide; SPION: superparamagnetic iron oxide nanoparticles; tPA: tissue plasminogen activator.
It is believed that MNPs are not toxic at low concentration, and their removal from the biological fluid can be allowed for by metabolic cascades.151 The factors which determine the biocompatibility and toxicity of MNPs are the existence of the magnetic components such as cobalt, iron, nickel, magnetite, and the final size, core and coating of the particles. Magnetite or its oxidized form is the most commonly employed NPs for biomedical applications. Highly magnetic compounds are toxic; hence, the compounds are of little interest. However, encapsulation enhances dispersibility, chemical stability, and declines toxicity. The major benefit of using NPs of sizes smaller than 100 nm is their enhanced tissular diffusion, lower sedimentation rates, and higher effective surface areas. Another benefit of using this type of NPs is a meaningful decline in the interactions of the magnetic dipole-dipole due to the compounds scale of r6.163-165 Table 3 summarizes the studies which have used organ-on-a-chip or 2D microfluidic models for drug delivery.
Table 3. Organ-on-a-chip and 2D microfluidic models for drug delivery .
| Cells | Culture | Nanoparticle | System | Brief summary of the study | Ref. |
| A549 | 2D dish culture | Cerium oxide NPs | - | Oxidative stress and cytotoxicity effects of CeO2 NPs on A549 cells | 166 |
| Human bronchoalveolar carcinoma | 2D dish culture | Silica NPs | - | The effect of Silica NPs size on toxicity in bronchoalveolar carcinoma | 167 |
| LCC6/Her2 | Droplet based microfluidics | DOX loaded CaCO3-NPs | In this system, the alginate beads were trapped in micro-sieve structures for cell culture in a continuous perfusion system. The environment permitted cell proliferation and the formation of multicellular spheroids. | Dose dependent cytotoxic effects of DOX loaded CaCO2-NPs | 168 |
| Human endothelial cell | 2D microfluidic channel | MSN | A simple microfluidic platform with precisely controlled shear stress conditions | Shear stress and endothelial cytotoxicity | 169 |
| MDA-MB-435 | Organ-on-a-chip (spheroid formation) | Gold NPs | A tumor-on-a-chip system where incorporation of tumor-like spheroids into a microfluidic channel permits real-time analysis of NP accumulation at physiological flow conditions. | Developing a tumor-on-a-chip system to study the transport of gold NPs through a 3D tissue environment and characterizing NPs within a tumor tissue. | 170 |
| Hy926 and human platelet | 2D microfluidic channel | FMS-NPs | The impact of sub-50 nm diameter mesoporous silica nanoparticles on platelet function is investigated using a microfluidic platform to model blood vessel characteristics | The effect of FMS-NPs on platelet aggregation | 171 |
| HUVEC | 2D microfluidic channel | Lipid-polymer NPs | Endothelialized microchip with controllable permeability can be used to probe nanoparticle translocation across an endothelial cell layer. | Microfluidic model of atherosclerosis to assessment of NPs endothelial translocation | 172 |
| MCF-7 and MVECs | Organ-on-a-chip (Pseudo 3D) | Fluorescent NPs | This model, consists of 3-dimensional microfluidic channels where tumor cells and endothelial cells are cultured within extracellular matrix under perfusion of interstitial fluid | Transport of NPs within the tumor | 173 |
| Caco-2, TH29-MTX and HepG2/C3A | 2D microfluidic channel | carboxylated polystyrene NPs | To construct this system, we combined in vitro models of the human intestinal epithelium, represented by a co-culture of Caco-2, TH29-MTX, and HepG2/C3A cells, within one microfluidic device. | Detection of liver injury using GI tract-liver-other tissue system | 174 |
| HUVEC | 2D microfluidic channel | Gold NPs | The tests performed in the microfluidic device were also run in multiwells, where no flow is present | Flow dependent cytotoxicity effects of gold NPs on endothelial | 175 |
| MCF-7, MDA-MB-231, and SUM-159PT | Organ-on-a-chip (3D gel pattering) | Dox-HANP | Three types of human breast cancer cell lines including MCF-7, MDA-MB-231, and SUM-159PT were cultured on a 3D platform, and their drug response and resistance to doxorubicin were characterized | The effects of NPs mediated drug delivery | 176 |
| HUVEC | 2D microfluidic channel | Gold NPs | A microfluidic device to observe how HUVEC viability changes when subject to a continuous flow of culture medium. | The effect of shear stress and gold NPs size on endothelial cytotoxicity | 177 |
| HUVEC | Organ-on-a-chip | HDL mimetic NPs | A micro-engineered three-dimensional vascular system | The effect of HDL mimetic NPs on endothelial cells | 178 |
CaCO3: calcium carbonate; CeO2: cerium oxide; DOX: doxorubicin; FMS: fluorescent mesoporous silica; MSN: mesoporous silica nanoparticle; HDL: high density lipoprotein; HANP: hyaluronic acid nanoparticle; gH625: membranotropic peptide; NPs: nanoparticles.
MNPs toxicity
There are some advantages in using MNPs for cancer diagnosis and treatment. On the contrary, due to some toxicity associated with the use of MNPs many restrictions have been identified in their application. Potential toxicity and some other features of MNPs are influenced by surface coatings of them. It has been illustrated in the research that there is positive relation in the size of the MNPs and accumulation. Therefore, if the size and surface coatings of MNPs have been controlled, it can reduce toxicity and improve magnetic behaviors. Currently, several magnetic materials with a broad spectrum of magnetic attributes are available. The high toxicity of some materials such as cobalt and chromium make them useless in biomedical applications. Such threats can be removed by a non- toxic coating which has high mechanical strength. Many studies have been conducted to develop different techniques for using Gadolinium NPs in medical imaging. Although the utilization of this compound for patients with renal failure is still controversial, Iron oxide NPs can be a good alternative for such patients. Due to the importance of the size in nanoparticles, the proportion of elements at the time when iron oxide is used as a contrast agent may be problematic, because the reason may lie in the fact that the size of the nanoparticles counts significantly. However, other types of paramagnetic and superparamagnetic NPs have been developed to overcome such drawbacks.163-165
Conclusion
NPs are self-assembled molecules which behave in a controlled manner. In some biomedical applications we need to use core shell MNPs to insure the biocompatibility and stability of biomolecules. In some biomedical applications we need to use core shell MNPs to insure the biocompatibility and stability of biomolecules. In some biomedical applications we need to use core shell MNPs to insure the biocompatibility and stability of biomolecules. In some biomedical applications we need to use core shell MNPs to insure the biocompatibility and stability of biomolecules. Nasopharyngeal carcinoma (NPC) was treated with cisplatin which was loaded on folate MNPs. Accordingly, they were targeted and accumulated in NPC cells efficiently. MNPs have some benefits for targeted drug delivery under image guidance which have been discussed in this review. We considered body magnetic fields to improve the efficiency and safety of drug delivery by using MNPs. Novel technologies such as tumor-on-a-chip platforms can offer other benefits along with in-vitro model which can be enjoyed in order to overcome and treat cancers. The combination of pharmacodynamics and pharmacokinetics NPs allow us to optimize the effectiveness of such treatment.
Ethical Issues
This article does not contain any studies with human and animal subjects performed by any of the authors.
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors are grateful to University of Kashan for supporting this work by Grant No. 785112.
References
- 1.Sun C, Lee JS, Zhang M. Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev. 2008;60(11):1252–65. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimi M, Wadajkar A, Subramanian K, Yousef M, Cui W, Hsieh JT. et al. In vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled drug delivery. Nanomedicine. 2010;6(5):672–80. doi: 10.1016/j.nano.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56(11):1649–59. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Blanco E, Kessinger CW, Sumer BD, Gao J. Multifunctional micellar nanomedicine for cancer therapy. Exp Biol Med (Maywood) 2009;234(2):123–31. doi: 10.3181/0808-mr-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serda RE, Adolphi NL, Bisoffi M, Sillerud LO. Targeting and cellular trafficking of magnetic nanoparticles for prostate cancer imaging. Mol Imaging. 2007;6(4):277–88. [PubMed] [Google Scholar]
- 6.Puech P, Huglo D, Petyt G, Lemaitre L, Villers A. Imaging of organ-confined prostate cancer: functional ultrasound, MRI and PET/computed tomography. Curr Opin Urol. 2009;19(2):168–76. doi: 10.1097/MOU.0b013e328323f5ed. [DOI] [PubMed] [Google Scholar]
- 7.He L, Wang M, Ge J, Yin Y. Magnetic assembly route to colloidal responsive photonic nanostructures. Acc Chem Res. 2012;45(9):1431–40. doi: 10.1021/ar200276t. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Yang Y, Ma W, Guo J, Lin Y, Wang C. Uniform magnetic core/shell microspheres functionalized with Ni2+-iminodiacetic acid for one step purification and immobilization of his-tagged enzymes. ACS Appl Mater Interfaces. 2013;5(7):2626–33. doi: 10.1021/am4006786. [DOI] [PubMed] [Google Scholar]
- 9.Sahoo SK, Ma W, Labhasetwar V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate cancer. Int J Cancer. 2004;112(2):335–40. doi: 10.1002/ijc.20405. [DOI] [PubMed] [Google Scholar]
- 10.Hattori Y, Ding WX, Maitani Y. Highly efficient cationic hydroxyethylated cholesterol-based nanoparticle-mediated gene transfer in vivo and in vitro in prostate carcinoma PC-3 cells. J Control Release. 2007;120(1-2):122–30. doi: 10.1016/j.jconrel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Douziech-Eyrolles L, Marchais H, Herve K, Munnier E, Souce M, Linassier C. et al. Nanovectors for anticancer agents based on superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2007;2(4):541–50. [PMC free article] [PubMed] [Google Scholar]
- 12.McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3(2):169–80. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Guo R, Yang M, Jiang X, Liu B. Thermo and pH dual‐responsive nanoparticles for anti‐cancer drug delivery. Adv Mater. 2007;19(19):2988–92. doi: 10.1002/adma.200601817. [DOI] [Google Scholar]
- 14.Soppimath KS, Tan DW, Yang YY. pH‐triggered thermally responsive polymer core–shell nanoparticles for drug delivery. Adv Mater. 2005;17(3):318–23. doi: 10.1002/adma.200401057. [DOI] [Google Scholar]
- 15.Wu X, Wang Z, Zhu D, Zong S, Yang L, Zhong Y. et al. pH and thermo dual-stimuli-responsive drug carrier based on mesoporous silica nanoparticles encapsulated in a copolymer-lipid bilayer. ACS Appl Mater Interfaces. 2013;5(21):10895–903. doi: 10.1021/am403092m. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Yu D, Jin C, Song X, Cheng J, Zhao X. et al. A dual responsive targeted drug delivery system based on smart polymer coated mesoporous silica for laryngeal carcinoma treatment. New J Chem. 2014;38(10):4830–6. doi: 10.1039/c4nj00579a. [DOI] [Google Scholar]
- 17.Yan K, Li P, Zhu H, Zhou Y, Ding J, Shen J. et al. Recent advances in multifunctional magnetic nanoparticles and applications to biomedical diagnosis and treatment. RSC Adv. 2013;3(27):10598–618. doi: 10.1039/c3ra40348c. [DOI] [Google Scholar]
- 18.Meng H, Xue M, Xia T, Zhao YL, Tamanoi F, Stoddart JF. et al. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J Am Chem Soc. 2010;132(36):12690–7. doi: 10.1021/ja104501a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ai H, Flask C, Weinberg B, Shuai XT, Pagel MD, Farrell D. et al. Magnetite‐loaded polymeric micelles as ultrasensitive magnetic‐resonance probes. Adv Mater. 2005;17(16):1949–52. doi: 10.1002/adma.200401904. [DOI] [Google Scholar]
- 20.Park JH, von Maltzahn G, Zhang L, Schwartz MP, Ruoslahti E, Bhatia SN. et al. Magnetic iron oxide nanoworms for tumor targeting and imaging. Adv Mater. 2008;20(9):1630–5. doi: 10.1002/adma.200800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolosnjaj-Tabi J, Di Corato R, Lartigue L, Marangon I, Guardia P, Silva AK. et al. Heat-generating iron oxide nanocubes: subtle “destructurators” of the tumoral microenvironment. ACS Nano. 2014;8(5):4268–83. doi: 10.1021/nn405356r. [DOI] [PubMed] [Google Scholar]
- 22.Yang X, Pilla S, Grailer JJ, Steeber DA, Gong S, Chen Y. et al. Tumor-targeting, superparamagnetic polymeric vesicles as highly efficient MRI contrast probes. J Mate Chem. 2009;19(32):5812–7. doi: 10.1039/B903845K. [DOI] [Google Scholar]
- 23.Yang J, Lee CH, Ko HJ, Suh JS, Yoon HG, Lee K. et al. Multifunctional magneto-polymeric nanohybrids for targeted detection and synergistic therapeutic effects on breast cancer. Angew Chem Int Ed Engl. 2007;46(46):8836–9. doi: 10.1002/anie.200703554. [DOI] [PubMed] [Google Scholar]
- 24.Sokol ES, Miller DH, Breggia A, Spencer KC, Arendt LM, Gupta PB. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016;18(1):19. doi: 10.1186/s13058-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Efremov AN, Stanganello E, Welle A, Scholpp S, Levkin PA. Micropatterned superhydrophobic structures for the simultaneous culture of multiple cell types and the study of cell-cell communication. Biomaterials. 2013;34(7):1757–63. doi: 10.1016/j.biomaterials.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CC, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol (Camb) 2014;6(6):603–10. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fong EL, Lamhamedi-Cherradi SE, Burdett E, Ramamoorthy V, Lazar AJ, Kasper FK. et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci U S A. 2013;110(16):6500–5. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esch EW, Bahinski A, Huh D. Organs-on-chips at the frontiers of drug discovery. Nat Rev Drug Discov. 2015;14(4):248–60. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi Y, Hyun E, Seo J, Blundell C, Kim HC, Lee E. et al. A microengineered pathophysiological model of early-stage breast cancer. Lab Chip. 2015;15(16):3350–7. doi: 10.1039/c5lc00514k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho MR, Lima D, Reis RL, Correlo VM, Oliveira JM. Evaluating biomaterial-and microfluidic-based 3D tumor models. Trends Biotechnol. 2015;33(11):667–78. doi: 10.1016/j.tibtech.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Peela N, Truong D, Saini H, Chu H, Mashaghi S, Ham SL. et al. Advanced biomaterials and microengineering technologies to recapitulate the stepwise process of cancer metastasis. Biomaterials. 2017;133:176–207. doi: 10.1016/j.biomaterials.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Young EW. Cells, tissues, and organs on chips: challenges and opportunities for the cancer tumor microenvironment. Integr Biol (Camb) 2013;5(9):1096–109. doi: 10.1039/c3ib40076j. [DOI] [PubMed] [Google Scholar]
- 33.Portillo-Lara R, Annabi N. Microengineered cancer-on-a-chip platforms to study the metastatic microenvironment. Lab Chip. 2016;16(21):4063–81. doi: 10.1039/c6lc00718j. [DOI] [PubMed] [Google Scholar]
- 34.Lee E, Song HG, Chen CS. Biomimetic on-a-chip platforms for studying cancer metastasis. Curr Opin Chem Eng. 2016;11:20–7. doi: 10.1016/j.coche.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaemmaghami AM, Hancock MJ, Harrington H, Kaji H, Khademhosseini A. Biomimetic tissues on a chip for drug discovery. Drug Discov Today. 2012;17(3-4):173–81. doi: 10.1016/j.drudis.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kashaninejad N, Nikmaneshi MR, Moghadas H, Kiyoumarsi Oskouei A, Rismanian M, Barisam M. et al. Organ-tumor-on-a-chip for chemosensitivity assay: A critical review. Micromachines (Basel) 2016;7(8) doi: 10.3390/mi7080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3(5):362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 38.Yung CW, Fiering J, Mueller AJ, Ingber DE. Micromagnetic-microfluidic blood cleansing device. Lab Chip. 2009;9(9):1171–7. doi: 10.1039/b816986a. [DOI] [PubMed] [Google Scholar]
- 39.Varshney A, Kumar G. Effects of magnetic field on cancer cell line. Journal of Experimental Biology and Agriculture Sciences. 2013;1(2S):91–6. [Google Scholar]
- 40.Caputa K, Dimbylow PJ, Dawson TW, Stuchly MA. Modelling fields induced in humans by 50/60 Hz magnetic fields: reliability of the results and effects of model variations. Phys Med Biol. 2002;47(8):1391–8. doi: 10.1088/0031-9155/47/8/311. [DOI] [PubMed] [Google Scholar]
- 41.Stuchly MA, Gandhi OP. Inter-laboratory comparison of numerical dosimetry for human exposure to 60 Hz electric and magnetic fields. Bioelectromagnetics. 2000;21(3):167–74. doi: 10.1002/(sici)1521-186x(200004)21:3<167::aid-bem3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Dimbylow P. Development of the female voxel phantom, NAOMI, and its application to calculations of induced current densities and electric fields from applied low frequency magnetic and electric fields. Phys Med Biol. 2005;50(6):1047–70. doi: 10.1088/0031-9155/50/6/002. [DOI] [PubMed] [Google Scholar]
- 43.Findlay RP, Lee AK, Dimbylow PJ. FDTD calculations of SAR for child voxel models in different postures between 10 MHz and 3 GHz. Radiat Prot Dosimetry. 2009;135(4):226–31. doi: 10.1093/rpd/ncp118. [DOI] [PubMed] [Google Scholar]
- 44.Gabriel C. Dielectric properties of biological tissue: variation with age. Bioelectromagnetics. 2005;Suppl 7:S12–8. doi: 10.1002/bem.20147. [DOI] [PubMed] [Google Scholar]
- 45.Dimbylow PJ. Current densities in a 2 mm resolution anatomically realistic model of the body induced by low frequency electric fields. Phys Med Biol. 2000;45(4):1013–22. doi: 10.1088/0031-9155/45/4/315. [DOI] [PubMed] [Google Scholar]
- 46.Bahr A, Bolz T, Hennes C. Numerical dosimetry ELF: accuracy of the method, variability of models and parameters, and the implication for quantifying guidelines. Health Phys. 2007;92(6):521–30. doi: 10.1097/01.HP.0000251249.00507.ca. [DOI] [PubMed] [Google Scholar]
- 47.Dawson TW, Caputa K, Stuchly MA. Influence of human model resolution on computed currents induced in organs by 60-Hz magnetic fields. Bioelectromagnetics. 1997;18(7):478–90. doi: 10.1002/(SICI)1521-186X(1997)18:7<478::AIDBEM3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 48.Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D. et al. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2006;130(6):1865–71. doi: 10.1053/j.gastro.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Jin X, Chalmers JJ, Zborowski M. Iron transport in cancer cell culture suspensions measured by cell magnetophoresis. Anal Chem. 2012;84(10):4520–6. doi: 10.1021/ac3004677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mutschke G, Tschulik K, Weier T, Uhlemann M, Bund A, Frohlich J. On the action of magnetic gradient forces in micro-structured copper deposition. Electrochim Acta. 2010;55(28):9060–6. doi: 10.1016/j.electacta.2010.08.046. [DOI] [Google Scholar]
- 51.Dunne P, Mazza L, Coey JM. Magnetic structuring of electrodeposits. Phys Rev Lett. 2011;107(2):024501. doi: 10.1103/PhysRevLett.107.024501. [DOI] [PubMed] [Google Scholar]
- 52.Zablotskii V, Dejneka A, Kubinova S, Le-Roy D, Dumas-Bouchiat F, Givord D. et al. Life on magnets: stem cell networking on micro-magnet arrays. PLoS One. 2013;8(8):e70416. doi: 10.1371/journal.pone.0070416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zablotskii V, Lunov O, Novotna B, Churpita O, Trosan P, Holan V. et al. Down-regulation of adipogenesis of mesenchymal stem cells by oscillating high-gradient magnetic fields and mechanical vibration. Appl Phys Lett. 2014;105(10):103702. doi: 10.1063/1.4895459. [DOI] [Google Scholar]
- 54.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montel F, Delarue M, Elgeti J, Malaquin L, Basan M, Risler T. et al. Stress clamp experiments on multicellular tumor spheroids. Phys Rev Lett. 2011;107(18):188102. doi: 10.1103/PhysRevLett.107.188102. [DOI] [PubMed] [Google Scholar]
- 56.Brem F, Hirt AM, Winklhofer M, Frei K, Yonekawa Y, Wieser HG. et al. Magnetic iron compounds in the human brain: a comparison of tumour and hippocampal tissue. J R Soc Interface. 2006;3(11):833–41. doi: 10.1098/rsif.2006.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teja AS, Koh PY. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Progress in Crystal Growth and Characterization of Materials. 2009;55(1-2):22–45. doi: 10.1016/j.pcrysgrow.2008.08.003. [DOI] [Google Scholar]
- 58.Mody VV, Siwale R, Singh A, Mody HR. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2(4):282–9. doi: 10.4103/0975-7406.72127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woinska M, Szczytko J, Majhofer A, Gosk J, Dziatkowski K, Twardowski A. Magnetic interactions in an ensemble of cubic nanoparticles: A Monte Carlo study. Phys Rev B. 2013;88(14):144421. doi: 10.1103/PhysRevB.88.144421. [DOI] [Google Scholar]
- 60.Banobre-Lopez M, Teijeiro A, Rivas J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep Pract Oncol Radiother. 2013;18(6):397–400. doi: 10.1016/j.rpor.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar CS, Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev. 2011;63(9):789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatterjee DK, Diagaradjane P, Krishnan S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther Deliv. 2011;2(8):1001–14. doi: 10.4155/tde.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 64.Yoo D, Jeong H, Noh SH, Lee JH, Cheon J. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew Chem Int Ed Engl. 2013;52(49):13047–51. doi: 10.1002/anie.201306557. [DOI] [PubMed] [Google Scholar]
- 65.Salunkhe AB, Khot VM, Pawar SH. Magnetic hyperthermia with magnetic nanoparticles: a status review. Curr Top Med Chem. 2014;14(5):572–94. doi: 10.2174/1568026614666140118203550. [DOI] [PubMed] [Google Scholar]
- 66.Jeon MJ, Ahn CH, Kim H, Chung IJ, Jung S, Kim YH. et al. The intratumoral administration of ferucarbotran conjugated with doxorubicin improved therapeutic effect by magnetic hyperthermia combined with pharmacotherapy in a hepatocellular carcinoma model. J Exp Clin Cancer Res. 2014;33:57. doi: 10.1186/s13046-014-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I, Hoopes PJ. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life. 2010;1(1n02) doi: 10.1142/s1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Revia RA, Zhang M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: recent advances. Mater Today (Kidlington) 2016;19(3):157–68. doi: 10.1016/j.mattod.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010;62(2):144–9. doi: 10.1016/j.phrs.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 70.Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew Chem Int Ed Engl. 2007;46(8):1222–44. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- 71.Li H, Parigi G, Luchinat C, Meade TJ. Bimodal Fluorescence-Magnetic Resonance Contrast Agent for Apoptosis Imaging. J Am Chem Soc. 2019;141(15):6224–33. doi: 10.1021/jacs.8b13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jacques V, Desreux JF. New classes of MRI contrast agents. In: Krause W, ed. Contrast Agents I: Magnetic Resonance Imaging. Berlin, Heidelberg: Springer; 2002:123-64.
- 73.Bullok KE, Gammon ST, Violini S, Prantner AM, Villalobos VM, Sharma V. et al. Permeation peptide conjugates for in vivo molecular imaging applications. Mol Imaging. 2006;5(1):1–15. [PubMed] [Google Scholar]
- 74.Nitin N, LaConte LE, Zurkiya O, Hu X, Bao G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. J Biol Inorg Chem. 2004;9(6):706–12. doi: 10.1007/s00775-004-0560-1. [DOI] [PubMed] [Google Scholar]
- 75.Qasba PK, Ramakrishnan B, Boeggeman E. Mutant glycosyltransferases assist in the development of a targeted drug delivery system and contrast agents for MRI. AAPS J. 2006;8(1):E190–5. doi: 10.1208/aapsj080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morishita N, Nakagami H, Morishita R, Takeda S, Mishima F, Terazono B. et al. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem Biophys Res Commun. 2005;334(4):1121–6. doi: 10.1016/j.bbrc.2005.06.204. [DOI] [PubMed] [Google Scholar]
- 77.Schillinger U, Brill T, Rudolph C, Huth S, Gersting S, Krotz F. et al. Advances in magnetofection--magnetically guided nucleic acid delivery. J Magn Magn Mater. 2005;293(1):501–8. doi: 10.1016/j.jmmm.2005.01.032. [DOI] [Google Scholar]
- 78.Yoon TJ, Kim JS, Kim BG, Yu KN, Cho MH, Lee JK. Multifunctional nanoparticles possessing a “magnetic motor effect” for drug or gene delivery. Angew Chem Int Ed Engl. 2005;44(7):1068–71. doi: 10.1002/anie.200461910. [DOI] [PubMed] [Google Scholar]
- 79.Gersting SW, Schillinger U, Lausier J, Nicklaus P, Rudolph C, Plank C. et al. Gene delivery to respiratory epithelial cells by magnetofection. J Gene Med. 2004;6(8):913–22. doi: 10.1002/jgm.569. [DOI] [PubMed] [Google Scholar]
- 80.Huth S, Lausier J, Gersting SW, Rudolph C, Plank C, Welsch U. et al. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J Gene Med. 2004;6(8):923–36. doi: 10.1002/jgm.577. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka H, Sugita T, Yasunaga Y, Shimose S, Deie M, Kubo T. et al. Efficiency of magnetic liposomal transforming growth factor-beta 1 in the repair of articular cartilage defects in a rabbit model. J Biomed Mater Res A. 2005;73(3):255–63. doi: 10.1002/jbm.a.30187. [DOI] [PubMed] [Google Scholar]
- 82.Kullberg M, Mann K, Owens JL. Improved drug delivery to cancer cells: a method using magnetoliposomes that target epidermal growth factor receptors. Med Hypotheses. 2005;64(3):468–70. doi: 10.1016/j.mehy.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 83.Cao J, Wang Y, Yu J, Xia J, Zhang C, Yin D. et al. Preparation and radiolabeling of surface-modified magnetic nanoparticles with rhenium-188 for magnetic targeted radiotherapy. J Magn Magn Mater. 2004;277(1-2):165–74. doi: 10.1016/j.jmmm.2003.10.022. [DOI] [Google Scholar]
- 84.Chen J, Wu H, Han D, Xie C. Using anti-VEGF McAb and magnetic nanoparticles as double-targeting vector for the radioimmunotherapy of liver cancer. Cancer Lett. 2006;231(2):169–75. doi: 10.1016/j.canlet.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 85.Alexiou C, Jurgons R, Schmid R, Hilpert A, Bergemann C, Parak F. et al. In vitro and in vivo investigations of targeted chemotherapy with magnetic nanoparticles. J Magn Magn Mater. 2005;293(1):389–93. doi: 10.1016/j.jmmm.2005.02.036. [DOI] [Google Scholar]
- 86.Jurgons R, Seliger C, Hilpert A, Trahms L, Odenbach S, Alexiou C. Drug loaded magnetic nanoparticles for cancer therapy. J Phys Condens Matter. 2006;18(38):S2893–S902. doi: 10.1088/0953-8984/18/38/s24. [DOI] [Google Scholar]
- 87.Hu FX, Neoh KG, Kang ET. Synthesis and in vitro anti-cancer evaluation of tamoxifen-loaded magnetite/PLLA composite nanoparticles. Biomaterials. 2006;27(33):5725–33. doi: 10.1016/j.biomaterials.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 88.Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang M. Methotrexate-immobilized poly (ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small. 2006;2(6):785–92. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]
- 89.Wu Y, Guo J, Yang W, Wang C, Fu S. Preparation and characterization of chitosan–poly (acrylic acid) polymer magnetic microspheres. Polymer. 2006;47(15):5287–94. doi: 10.1016/j.polymer.2006.05.017. [DOI] [Google Scholar]
- 90.Port RE, Schuster C, Port CR, Bachert P. Simultaneous sustained release of fludarabine monophosphate and Gd-DTPA from an interstitial liposome depot in rats: potential for indirect monitoring of drug release by magnetic resonance imaging. Cancer Chemother Pharmacol. 2006;58(5):607–17. doi: 10.1007/s00280-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 91.Yang J, Lee H, Hyung W, Park SB, Haam S. Magnetic PECA nanoparticles as drug carriers for targeted delivery: synthesis and release characteristics. J Microencapsul. 2006;23(2):203–12. doi: 10.1080/02652040500435444. [DOI] [PubMed] [Google Scholar]
- 92.Kohler N, Sun C, Wang J, Zhang M. Methotrexate-modified superparamagnetic nanoparticles and their intracellular uptake into human cancer cells. Langmuir. 2005;21(19):8858–64. doi: 10.1021/la0503451. [DOI] [PubMed] [Google Scholar]
- 93.Cheung RY, Ying Y, Rauth AM, Marcon N, Yu Wu X. Biodegradable dextran-based microspheres for delivery of anticancer drug mitomycin C. Biomaterials. 2005;26(26):5375–85. doi: 10.1016/j.biomaterials.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 94.Roeth AA, Slabu I, Baumann M, Alizai PH, Schmeding M, Guentherodt G. et al. Establishment of a biophysical model to optimize endoscopic targeting of magnetic nanoparticles for cancer treatment. Int J Nanomedicine. 2017;12:5933–40. doi: 10.2147/ijn.s132162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang JQ, Zhang ZR, Yang H, Tan QY, Qin SR, Qiu XL. Lyophilized paclitaxel magnetoliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: in vitro and in vivo studies. Pharm Res. 2005;22(4):573–83. doi: 10.1007/s11095-005-2496-8. [DOI] [PubMed] [Google Scholar]
- 96.Saravanan M, Bhaskar K, Maharajan G, Pillai KS. Ultrasonically controlled release and targeted delivery of diclofenac sodium via gelatin magnetic microspheres. Int J Pharm. 2004;283(1-2):71–82. doi: 10.1016/j.ijpharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 97.Jain S, Mishra V, Singh P, Dubey PK, Saraf DK, Vyas SP. RGD-anchored magnetic liposomes for monocytes/neutrophils-mediated brain targeting. Int J Pharm. 2003;261(1-2):43–55. doi: 10.1016/s0378-5173(03)00269-2. [DOI] [PubMed] [Google Scholar]
- 98.Kubo T, Sugita T, Shimose S, Nitta Y, Ikuta Y, Murakami T. Targeted systemic chemotherapy using magnetic liposomes with incorporated adriamycin for osteosarcoma in hamsters. Int J Oncol. 2001;18(1):121–5. doi: 10.3892/ijo.18.1.121. [DOI] [PubMed] [Google Scholar]
- 99.Mody VV, Cox A, Shah S, Singh A, Bevins W, Parihar H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl Nanosci. 2014;4(4):385–92. doi: 10.1007/s13204-013-0216-y. [DOI] [Google Scholar]
- 100.Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys. 2003;36(13):R167–81. doi: 10.1088/0022-3727/36/13/201. [DOI] [Google Scholar]
- 101.Baghi M, Mack MG, Wagenblast J, Hambek M, Rieger J, Bisdas S. et al. Iron oxide particle-enhanced magnetic resonance imaging for detection of benign lymph nodes in the head and neck: how reliable are the results? Anticancer Res. 2007;27(5b):3571–5. [PubMed] [Google Scholar]
- 102.Sadat U, Usman A, Gillard JH. Imaging pathobiology of carotid atherosclerosis with ultrasmall superparamagnetic particles of iron oxide: an update. Curr Opin Cardiol. 2017;32(4):437–40. doi: 10.1097/hco.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reimer P, Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur Radiol. 2003;13(6):1266–76. doi: 10.1007/s00330-002-1721-7. [DOI] [PubMed] [Google Scholar]
- 104.Wang YX, Hussain SM, Krestin GP. Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol. 2001;11(11):2319–31. doi: 10.1007/s003300100908. [DOI] [PubMed] [Google Scholar]
- 105.Michel SC, Keller TM, Frohlich JM, Fink D, Caduff R, Seifert B. et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology. 2002;225(2):527–36. doi: 10.1148/radiol.2252011605. [DOI] [PubMed] [Google Scholar]
- 106.Trivedi RA, Mallawarachi C, JM UK, Graves MJ, Horsley J, Goddard MJ. et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26(7):1601–6. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 107.Tartaj P, Morales MD, Veintemillas-Verdaguer S, Gonzalez-Carreno T, Serna CJ. The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36(13):R182–97. doi: 10.1088/0022-3727/36/13/202. [DOI] [Google Scholar]
- 108.Laurent S, Saei AA, Behzadi S, Panahifar A, Mahmoudi M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv. 2014;11(9):1449–70. doi: 10.1517/17425247.2014.924501. [DOI] [PubMed] [Google Scholar]
- 109.Schwenk MH. Ferumoxytol: a new intravenous iron preparation for the treatment of iron deficiency anemia in patients with chronic kidney disease. Pharmacotherapy. 2010;30(1):70–9. doi: 10.1592/phco.30.1.70. [DOI] [PubMed] [Google Scholar]
- 110.Obaidat IM, Issa B, Haik Y. Magnetic properties of magnetic nanoparticles for efficient hyperthermia. Nanomaterials. 2015;5(1):63–89. doi: 10.3390/nano5010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hughes GA. Nanostructure-mediated drug delivery. Nanomedicine. 2005;1(1):22–30. doi: 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 112.Singh SK, Singh S, Lillard JW Jr, Singh R. Drug delivery approaches for breast cancer. Int J Nanomedicine. 2017;12:6205–18. doi: 10.2147/ijn.s140325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Konar D, Devarasetty M, Yildiz DV, Atala A, Murphy SV. Lung-on-a-chip technologies for disease modeling and drug development. Biomed Eng Comput Biol. 2016;7(Suppl 1):17–27. doi: 10.4137/becb.s34252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gelperina S, Kisich K, Iseman MD, Heifets L. The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med. 2005;172(12):1487–90. doi: 10.1164/rccm.200504-613PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu J, Dong M, Rigatto C, Liu Y, Lin F. Lab-on-chip technology for chronic disease diagnosis. NPJ Digit Med. 2018;1(1):7. doi: 10.1038/s41746-017-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bielecka MK, Tezera LB, Zmijan R, Drobniewski F, Zhang X, Jayasinghe S. et al. A bioengineered three-dimensional cell culture platform integrated with microfluidics to address antimicrobial resistance in tuberculosis. MBio. 2017;8(1):e02073–16. doi: 10.1128/mBio.02073-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abu-Dief AM, Abdel-Fatah SM. Development and functionalization of magnetic nanoparticles as powerful and green catalysts for organic synthesis. Beni-Suef Univ J Basic Appl Sci. 2018;7(1):55–67. doi: 10.1016/j.bjbas.2017.05.008. [DOI] [Google Scholar]
- 118.Dobson J. Magnetic nanoparticles for drug delivery. Drug Dev Res. 2006;67(1):55–60. doi: 10.1002/ddr.20067. [DOI] [Google Scholar]
- 119.Krukemeyer MG, Krenn V, Jakobs M, Wagner W. Magnetic drug targeting in a rhabdomyosarcoma rat model using magnetite-dextran composite nanoparticle-bound mitoxantrone and 06 tesla extracorporeal magnets - sarcoma treatment in progress. J Drug Target. 2012;20(2):185–93. doi: 10.3109/1061186x.2011.622399. [DOI] [PubMed] [Google Scholar]
- 120.Kumar M, Yigit M, Dai G, Moore A, Medarova Z. Image-guided breast tumor therapy using a small interfering RNA nanodrug. Cancer Res. 2010;70(19):7553–61. doi: 10.1158/0008-5472.can-10-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li Z, Dong K, Huang S, Ju E, Liu Z, Yin M. et al. A smart nanoassembly for multistage targeted drug delivery and magnetic resonance imaging. Adv Funct Mater. 2014;24(23):3612–20. doi: 10.1002/adfm.201303662. [DOI] [Google Scholar]
- 122.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B. et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103(2):317–24. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chauhan VP, Stylianopoulos T, Martin JD, Popovic Z, Chen O, Kamoun WS. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–8. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I. et al. Investigating the optimal size of anticancer nanomedicine. Proc Natl Acad Sci U S A. 2014;111(43):15344–9. doi: 10.1073/pnas.1411499111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arruebo M, Fernandez-Pacheco R, Ibarra MR, Santamaria J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2(3):22–32. doi: 10.1016/S1748-0132(07)70084-1. [DOI] [Google Scholar]
- 126.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 127.Peng XH, Qian X, Mao H, Wang AY, Chen ZG, Nie S. et al. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int J Nanomedicine. 2008;3(3):311–21. doi: 10.2147/ijn.s2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.El-Boubbou K. Magnetic iron oxide nanoparticles as drug carriers: clinical relevance. Nanomedicine (Lond) 2018;13(8):953–71. doi: 10.2217/nnm-2017-0336. [DOI] [PubMed] [Google Scholar]
- 129. Lowry GV, Gregory KB, Apte SC, Lead JR. Transformations of nanomaterials in the environment. ACS Publications; 2012. [DOI] [PubMed]
- 130.Kolosnjaj-Tabi J, Lartigue L, Javed Y, Luciani N, Pellegrino T, Wilhelm C. et al. Biotransformations of magnetic nanoparticles in the body. Nano Today. 2016;11(3):280–4. doi: 10.1016/j.nantod.2015.10.001. [DOI] [Google Scholar]
- 131.Soenen SJ, Parak WJ, Rejman J, Manshian B. (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem Rev. 2015;115(5):2109–35. doi: 10.1021/cr400714j. [DOI] [PubMed] [Google Scholar]
- 132.Loeve S, Vincent BB, Gazeau F. Nanomedicine metaphors: From war to care Emergence of an oecological approach. Nano Today. 2013;8(6):560–5. doi: 10.1016/j.nantod.2013.08.003. [DOI] [Google Scholar]
- 133.Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part Fibre Toxicol. 2012;9:20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bertoli F, Davies GL, Monopoli MP, Moloney M, Gun’ko YK, Salvati A. et al. Magnetic nanoparticles to recover cellular organelles and study the time resolved nanoparticle-cell interactome throughout uptake. Small. 2014;10(16):3307–15. doi: 10.1002/smll.201303841. [DOI] [PubMed] [Google Scholar]
- 135.Levy M, Luciani N, Alloyeau D, Elgrabli D, Deveaux V, Pechoux C. et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials. 2011;32(16):3988–99. doi: 10.1016/j.biomaterials.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 136.Lartigue L, Alloyeau D, Kolosnjaj-Tabi J, Javed Y, Guardia P, Riedinger A. et al. Biodegradation of iron oxide nanocubes: high-resolution in situ monitoring. ACS Nano. 2013;7(5):3939–52. doi: 10.1021/nn305719y. [DOI] [PubMed] [Google Scholar]
- 137.Caballero D, Kaushik S, Correlo VM, Oliveira JM, Reis RL, Kundu SC. Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials. 2017;149:98–115. doi: 10.1016/j.biomaterials.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 138.Asghar W, El Assal R, Shafiee H, Pitteri S, Paulmurugan R, Demirci U. Engineering cancer microenvironments for in vitro 3-D tumor models. Mater Today (Kidlington) 2015;18(10):539–53. doi: 10.1016/j.mattod.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Couvreur P. Nanoparticles in drug delivery: past, present and future. Adv Drug Deliv Rev. 2013;65(1):21–3. doi: 10.1016/j.addr.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 140.Zhang YS, Zhang YN, Zhang W. Cancer-on-a-chip systems at the frontier of nanomedicine. Drug Discov Today. 2017;22(9):1392–9. doi: 10.1016/j.drudis.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alemany-Ribes M, Semino CE. Bioengineering 3D environments for cancer models. Adv Drug Deliv Rev. 2014;79-80:40–9. doi: 10.1016/j.addr.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 142.Wu M, Swartz MA. Modeling tumor microenvironments in vitro. J Biomech Eng. 2014;136(2):021011. doi: 10.1115/1.4026447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hielscher AC, Gerecht S. Engineering approaches for investigating tumor angiogenesis: exploiting the role of the extracellular matrix. Cancer Res. 2012;72(23):6089–96. doi: 10.1158/0008-5472.can-12-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483(7391):531–3. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 145.Buchanan CF, Voigt EE, Szot CS, Freeman JW, Vlachos PP, Rylander MN. Three-dimensional microfluidic collagen hydrogels for investigating flow-mediated tumor-endothelial signaling and vascular organization. Tissue Eng Part C Methods. 2014;20(1):64–75. doi: 10.1089/ten.TEC.2012.0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jeong SY, Lee JH, Shin Y, Chung S, Kuh HJ. Co-culture of tumor spheroids and fibroblasts in a collagen matrix-incorporated microfluidic chip mimics reciprocal activation in solid tumor microenvironment. PLoS One. 2016;11(7):e0159013. doi: 10.1371/journal.pone.0159013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tsai HF, Trubelja A, Shen AQ, Bao G. Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment. J R Soc Interface. 2017;14(131) doi: 10.1098/rsif.2017.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Benhal P, Broda A, Najafali D, Malik P, Mohammed A, Ramaswamy B. et al. On-chip testing of the speed of magnetic nano- and micro-particles under a calibrated magnetic gradient. J Magn Magn Mater. 2019;474:187–98. doi: 10.1016/j.jmmm.2018.10.148. [DOI] [Google Scholar]
- 149.Geczy R, Agnoletti M, Hansen MF, Kutter JP, Saatchi K, Hafeli UO. Microfluidic approaches for the production of monodisperse, superparamagnetic microspheres in the low micrometer size range. J Magn Magn Mater. 2019;471:286–93. doi: 10.1016/j.jmmm.2018.09.091. [DOI] [Google Scholar]
- 150.Yoo D, Lee JH, Shin TH, Cheon J. Theranostic magnetic nanoparticles. Acc Chem Res. 2011;44(10):863–74. doi: 10.1021/ar200085c. [DOI] [PubMed] [Google Scholar]
- 151.Malik A, Butt TT, Zahid S, Zahid F, Waquar S, Rasool M. et al. Use of magnetic nanoparticles as targeted therapy: theranostic approach to treat and diagnose cancer. J Nanotechnol. 2017;2017:1098765. doi: 10.1155/2017/1098765. [DOI] [Google Scholar]
- 152.Shevtsov M, Multhoff G. Recent developments of magnetic nanoparticles for theranostics of brain tumor. Curr Drug Metab. 2016;17(8):737–44. doi: 10.2174/1389200217666160607232540. [DOI] [PubMed] [Google Scholar]
- 153.Luo Y, Huang L, Yang Y, Zhuang X, Hu S, Ju H. et al. A programmed nanoparticle with self-adapting for accurate cancer cell eradication and therapeutic self-reporting. Theranostics. 2017;7(5):1245–56. doi: 10.7150/thno.18187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sarkar SK, Khater Y, Kulkarni A, Sengupta S. Feedback-mediated cancer therapy: a FRET-based nanoreporter approach Biosensing and Nanomedicine VII; 2014: International Society for Optics and Photonics. Proceedings of the SPIE. 2014 doi: 10.1117/12.2061379. [DOI] [Google Scholar]
- 155.Choi KY, Jeon EJ, Yoon HY, Lee BS, Na JH, Min KH. et al. Theranostic nanoparticles based on PEGylated hyaluronic acid for the diagnosis, therapy and monitoring of colon cancer. Biomaterials. 2012;33(26):6186–93. doi: 10.1016/j.biomaterials.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song J, Yang X, Yang Z, Lin L, Liu Y, Zhou Z. et al. Rational design of branched nanoporous gold nanoshells with enhanced physico-optical properties for optical imaging and cancer therapy. ACS Nano. 2017;11(6):6102–13. doi: 10.1021/acsnano.7b02048. [DOI] [PubMed] [Google Scholar]
- 157.Guo Y, Chen W, Wang W, Shen J, Guo R, Gong F. et al. Simultaneous diagnosis and gene therapy of immuno-rejection in rat allogeneic heart transplantation model using a T-cell-targeted theranostic nanosystem. ACS Nano. 2012;6(12):10646–57. doi: 10.1021/nn3037573. [DOI] [PubMed] [Google Scholar]
- 158.Crisci E, Fraile L, Novellas R, Espada Y, Cabezon R, Martinez J. et al. In vivo tracking and immunological properties of pulsed porcine monocyte-derived dendritic cells. Mol Immunol. 2015;63(2):343–54. doi: 10.1016/j.molimm.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 159.Gao S, Wang G, Qin Z, Wang X, Zhao G, Ma Q. et al. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials. 2017;112:324–35. doi: 10.1016/j.biomaterials.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 160.Kempen PJ, Greasley S, Parker KA, Campbell JL, Chang HY, Jones JR. et al. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics. 2015;5(6):631–42. doi: 10.7150/thno.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li Z, Jin Q, Huang C, Dasa S, Chen L, Yap LP. et al. Trackable and targeted phage as positron emission tomography (PET) agent for cancer imaging. Theranostics. 2011;1:371–80. doi: 10.7150/thno/v01p0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim JY, Ryu JH, Schellingerhout D, Sun IC, Lee SK, Jeon S. et al. Direct imaging of cerebral thromboemboli using computed tomography and fibrin-targeted gold nanoparticles. Theranostics. 2015;5(10):1098–114. doi: 10.7150/thno.11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B. et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lee SJ, Jeong JR, Shin SC, Kim JC, Chang YH, Chang YM. et al. Nanoparticles of magnetic ferric oxides encapsulated with poly (D, L latide-co-glycolide) and their applications to magnetic resonance imaging contrast agent. J Magn Magn Mater. 2004;272-276:2432–3. doi: 10.1016/j.jmmm.2003.12.416. [DOI] [Google Scholar]
- 165.Park J, Lee E, Hwang NM, Kang M, Kim SC, Hwang Y. et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew Chem Int Ed Engl. 2005;44(19):2873–7. doi: 10.1002/anie.200461665. [DOI] [PubMed] [Google Scholar]
- 166.Lin W, Huang YW, Zhou XD, Ma Y. Toxicity of cerium oxide nanoparticles in human lung cancer cells. Int J Toxicol. 2006;25(6):451–7. doi: 10.1080/10915810600959543. [DOI] [PubMed] [Google Scholar]
- 167.Lin W, Huang YW, Zhou XD, Ma Y. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol Appl Pharmacol. 2006;217(3):252–9. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 168.Yu L, Chen MC, Cheung KC. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip. 2010;10(18):2424–32. doi: 10.1039/c004590j. [DOI] [PubMed] [Google Scholar]
- 169.Kim D, Lin YS, Haynes CL. On-chip evaluation of shear stress effect on cytotoxicity of mesoporous silica nanoparticles. Anal Chem. 2011;83(22):8377–82. doi: 10.1021/ac202115a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Albanese A, Lam AK, Sykes EA, Rocheleau JV, Chan WC. Tumour-on-a-chip provides an optical window into nanoparticle tissue transport. Nat Commun. 2013;4:2718. doi: 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kim D, Finkenstaedt-Quinn S, Hurley KR, Buchman JT, Haynes CL. On-chip evaluation of platelet adhesion and aggregation upon exposure to mesoporous silica nanoparticles. Analyst. 2014;139(5):906–13. doi: 10.1039/c3an01679j. [DOI] [PubMed] [Google Scholar]
- 172.Kim Y, Lobatto ME, Kawahara T, Lee Chung B, Mieszawska AJ, Sanchez-Gaytan BL. et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci U S A. 2014;111(3):1078–83. doi: 10.1073/pnas.1322725111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kwak B, Ozcelikkale A, Shin CS, Park K, Han B. Simulation of complex transport of nanoparticles around a tumor using tumor-microenvironment-on-chip. J Control Release. 2014;194:157–67. doi: 10.1016/j.jconrel.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Esch MB, Mahler GJ, Stokol T, Shuler ML. Body-on-a-chip simulation with gastrointestinal tract and liver tissues suggests that ingested nanoparticles have the potential to cause liver injury. Lab Chip. 2014;14(16):3081–92. doi: 10.1039/c4lc00371c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fede C, Fortunati I, Weber V, Rossetto N, Bertasi F, Petrelli L. et al. Evaluation of gold nanoparticles toxicity towards human endothelial cells under static and flow conditions. Microvasc Res. 2015;97:147–55. doi: 10.1016/j.mvr.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 176.Shin K, Klosterhoff BS, Han B. Characterization of cell-type-specific drug transport and resistance of breast cancers using tumor-microenvironment-on-chip. Mol Pharm. 2016;13(7):2214–23. doi: 10.1021/acs.molpharmaceut.6b00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fede C, Albertin G, Petrelli L, De Caro R, Fortunati I, Weber V. et al. Influence of shear stress and size on viability of endothelial cells exposed to gold nanoparticles. J Nanopart Res. 2017;19(9):316. doi: 10.1007/s11051-017-3993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ahn J, Sei YJ, Jeon NL, Kim Y. Probing the effect of bioinspired nanomaterials on angiogenic sprouting with a microengineered vascular system. IEEE Trans Nanotechnol. 2018;17(3):393–7. doi: 10.1109/TNANO.2017.2771426. [DOI] [Google Scholar]


