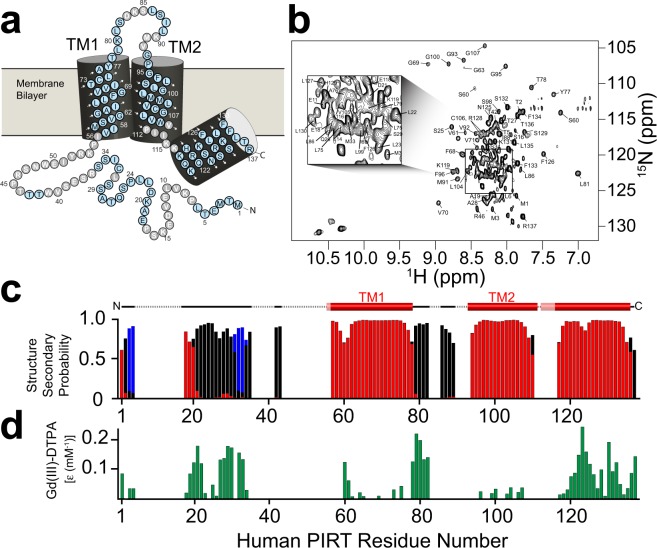
Figure 1.
NMR derived secondary structure and topology of hPIRT. (a) The light blue (gray) colored circles indicate NMR assigned (unassigned) residues. (b) The TROSY-HSQC of hPIRT reconstituted in DPC shows a relatively well-resolved spectrum with dispersion consistent with a α-helical membrane protein. (c) The plot of the consensus TALOS-N predicted hPIRT secondary structure derived from experimental hPIRT Cα, Cβ, and C′ chemical shifts. Red, black, and blue bars indicate the probability of α-helix, loop, and β-sheet respectively. (d) Solvent paramagnetic enhancement in spin relaxation, ε, from Gd(III)-DTPA confirm the hydrophobic nature of the two transmembrane α-helices and the amphipathic nature of the C-terminal α-helix. Larger magnitude values of ε are consistent with solvent accessibility and small magnitude ε values are suggestive of protection from Gd(III)-DTPA relaxation enhancement by the membrane mimic. These results indicate that hPIRT has a relatively unstructured N-terminus, two transmembrane helices, and an amphipathic C-terminal α-helix.