Figure 1.
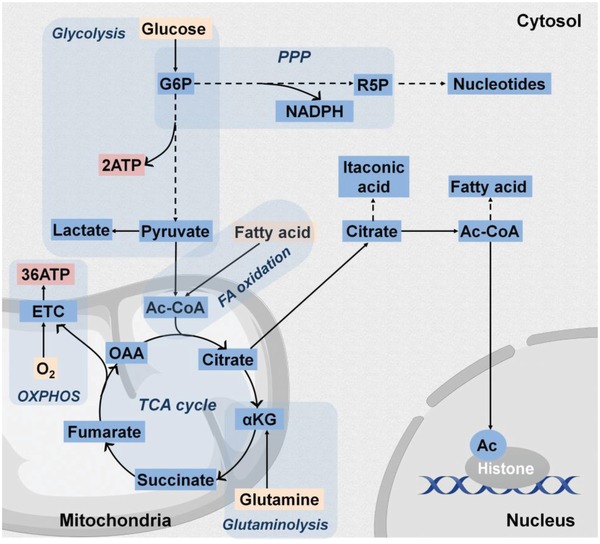
Overview of the core cell metabolic pathways. The energy source, ATP, is imperative for cell survival, proliferation, differentiation, and cell‐specific functions. ATP generation is derived from the intracellular processes, glycolysis, and cellular respiration. Glucose, a primary energy substrate, is imported into the cytosol, from the extracellular space and undergoes conversion into pyruvate via a series of chemical reactions collectively known as glycolysis obtaining a net of 2 ATP per mole of glucose. Pyruvate is converted into acetyl–CoA (Ac–CoA) in mitochondrial to enter the tricarboxylic acid (TCA) cycle, which drives the electron transfer chain (ETC) yielding a net production of 36 ATP molecules in a process called oxidative phosphorylation (OXPHOS). Notably, in certain cell types, such as cancer and endothelial cells, glycolysis‐derived pyruvate molecules are converted into lactate even when ample O2 is available, a phenomenon referred to as aerobic glycolysis. The glycolytic flux can also be directed through the pentose phosphate pathway (PPP) to generate NADPH, a cofactor for redox homeostasis and cellular respiration, as well as ribose‐5‐phosphate, a substrate for nucleotide synthesis. Furthermore, alternative macromolecules can feed into the TCA cycle besides pyruvate exhibited by conversion of fatty acids to acetyl–CoA via β‐oxidation and by conversion of glutamine to α‐ketoglutarate (α‐KG) via glutaminolysis. TCA cycle citrate may also be exported to the cytosol where it serves as a substrate for itaconic acid synthesis in M1 macrophages or may be converted to acetyl–CoA for fatty acid synthesis. Nucleocytoplasmic acetyl–CoA is additionally required as a substrate for histone acetylation (Ac) of chromatin histones, which impacts chromatin structure and gene transcription.
