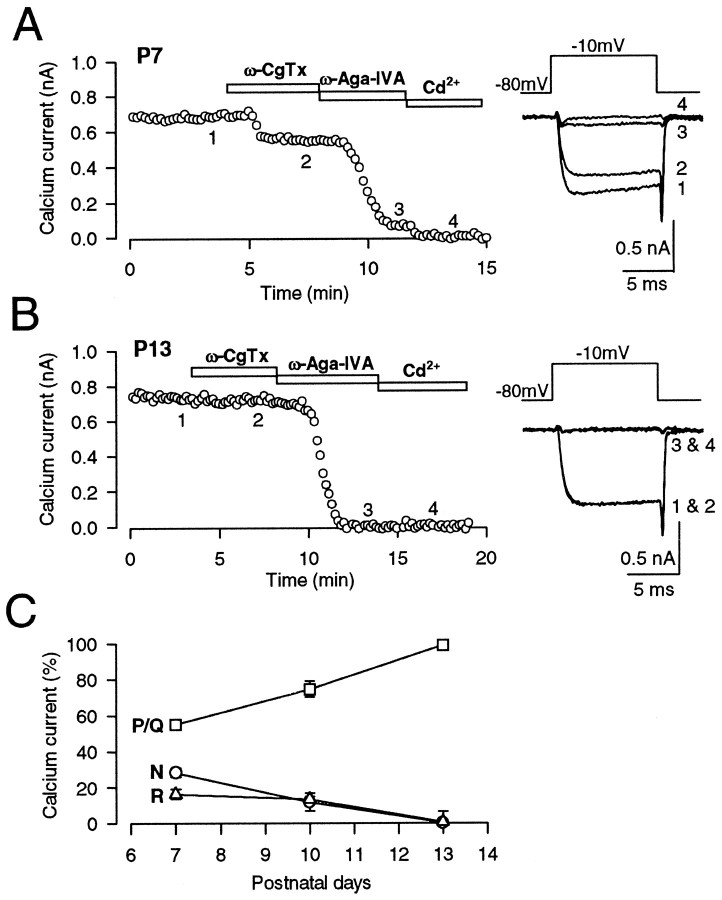
Fig. 3.
Developmental decline of N- and R-type Ca2+ channels in the giant presynaptic terminal, the calyx of Held. Presynaptic Ca2+ currents (IpCa) were evoked by a 10 msec depolarizing pulse from −80 mV holding potential to −10 mV under voltage clamp every 10 sec in the presence of TTX (0.1 μm) and TEA-Cl (10 mm). A, At P7, ω-CgTx (3 μm) reduced the amplitude ofIpCa by 23% (2), whereas ω-Aga-IVA (200 nm) by 66% (3). The fraction remaining after application of both toxins (11%) was abolished by Cd2+ (100 μm; 4). B, At P13, ω-CgTx had no effect on IpCa, whereas ω-Aga-IVA almost completely abolishedIpCa (3) with no appreciable remaining Cd2+-sensitive component (4). C, The fraction ofIpCa blocked by ω-CgTx (N, ○), ω-Aga-IVA (P/Q, ■), and that insensitive to the toxins but blocked by Cd2+ (R, ▵) at three different postnatal ages. Symbols and error bars derived from five to eight cells at each age.