Figure 2.
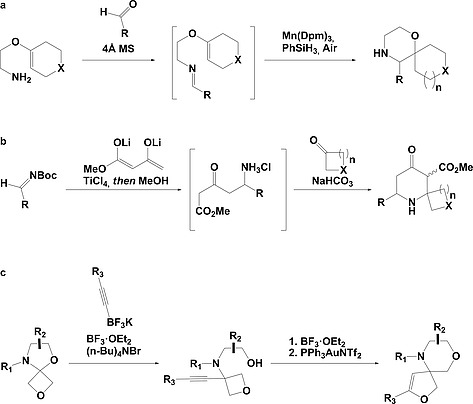
Recent advances in the synthesis of heterocyclic spirocycles (a) Wang and Bode generated unactivated imines and utilised metal hydride hydrogen atom transfer chemistry with manganese to generate a C‐centered radical, which underwent addition to the unactivated imine to generate the spirocycle with an N‐centered radical from which the unprotected‐N was unveiled by a second hydrogen atom transfer: 19 spirocycles with R = alkyl or aryl; X = NCbz, S or CH2; n = 5 or 6;46 (b) Griggs et al. formed highly substituted 2‐spiropiperidines in a one‐pot reaction from N‐Boc imines via the addition of Chan's diene under Maitland–Japp conditions followed by in‐situ Boc‐deprotection and a sodium bicarbonate catalysed cyclisation onto a cyclic ketone: 10 spirocycles with R = alkyl or aryl; X = NCbz, O, S or CH2; n = 4, 5 or 6;47 (c) Brady and Carreira used the addition of trifluoroborates to oxetanyl N,O‐acetals to generate products with oxetane and alkyne functionalities from which spiro heterocycles can be generated by orthogonal activation, such as intramolecular opening of the oxetane by the alcohol followed by catalytic intramolecular alkyne hydroalkoxylation to close the spirocyclic ring: 5 spirocycles with R = alkyl, aryl or TMS.42
