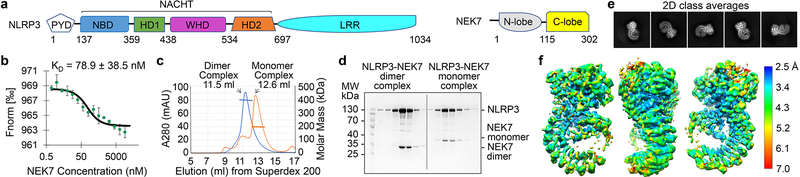
Fig. 1 |. Biochemical characterization and cryo-EM structure determination.
a, Schematic domain representation of human NLRP3 and NEK7, with labelled domain boundary defined from this work. b, MST analysis of NEK7 binding to NLRP3. A dissociation constant of 78.9 ± 38.5 nM was calculated from three independent replicates (shown as mean ± s.d.). c, Reconstitution of NLRP3-NEK7 dimer and monomer complexes on Superdex 200 gel filtration column (repeated ≥ 5 times). Molecular mass distribution within each peak was calculated from in-line MALS measurements, and shown in blue and orange for the dimer and monomer complexes, respectively (Performed once). d, SDS-PAGE gels of eluted fractions of dimer and monomer complexes (repeated ≥ 5 times). e, Representative 2D class averages from the 300 keV cryo-EM dataset, selected amongst 100 classes. f, The final cryo-EM density map (shown at 6σ) in three orientations and coloured with local resolutions by ResMap48.