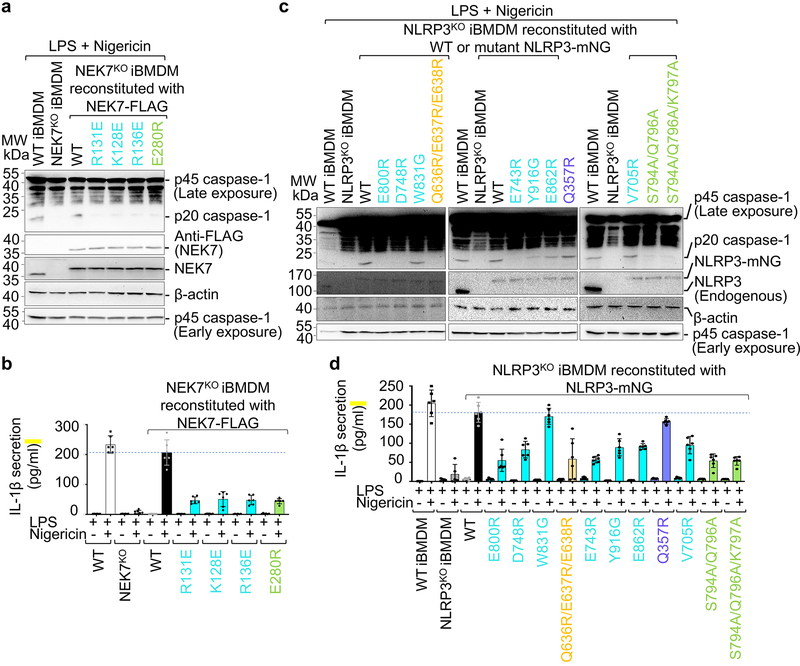
Fig. 4 |. Structure-guided mutations of NLRP3 and NEK7 on inflammasome activation.
NEK7 and NLRP3 mutations are coloured as in Fig. 3d–e except that mutations on interactions in trans are coloured in green. a-b, NEK7KO iBMDMs were reconstituted with WT or mutant FLAG-tagged human NEK7, primed by LPS (4 hours) and stimulated by nigericin (30 min). Cells were analysed by Western blot for caspase-1 processing using a specific anti-caspase-1 antibody (repeated 3 times) (a). The full-length caspase-1 (p45) and processed large subunit of caspase-1 (p20) are labelled (first blot from the top). Caspase-1 p45 was also analysed by an early exposure to show equal loading (fifth blot). Reconstituted NEK7 was probed by anti-NEK7 and anti-FLAG antibodies (second and third blots), and loading was analysed by an anti-β-actin antibody (fourth blot). Mature IL-1β released in the supernatant was measured by ELISA (b). Data are presented as mean ± s.d. for n=3 each from two independent experiments. Dots: individual data points. c-d, NLRP3KO iBMDMs were reconstituted with WT or mutant human mNeonGreen-tagged NLRP3, primed by LPS (4 hours) and stimulated by nigericin (30 min). Caspase-1 processing (repeated 3 times) (c) and IL-1β release (shown as mean ± s.d. with n=3 each from two independent experiments) (d) were analysed as in (a-b). Dots: individual data points. For gel source data, see Supplementary Figure 1.