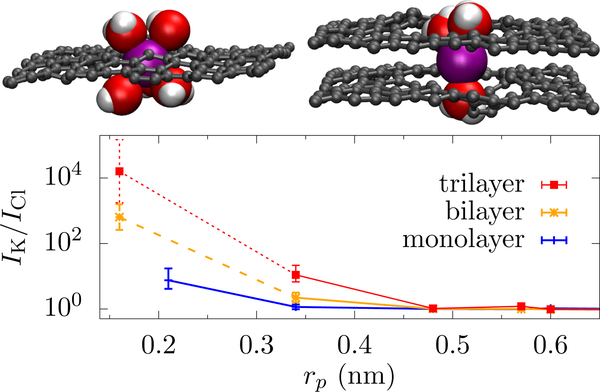
FIG. 9.
K+ (purple) translocating through mono- (left) and bi-layer (right) graphene pores (geometric radii of 0.2 nm and 0.16 nm, respectively). The carbon atoms are shown as smaller gray spheres (not the vdW radii like the other atoms) along with the carbon-carbon bond. For pores of this size, ions cannot retain the complete hydration shell when translocating. For monolayer graphene, K+ loses roughly two water molecules from its first hydration shell but still retains four closely bound water molecules just outside the membrane [large red (O) and small white (H) spheres]. For bilayer graphene, however, water molecules can hydrate only on the “two ends” of the ion, which gives a substantially larger energy barrier. Tri-layer graphene further limits hydration. The bottom panel shows the K+ over Cl− selectivity (given by the ratio of their currents, IK/ICl) in graphene pores versus the geometric radius. The multilayer graphene is AB stacked, which influences the allowed radii. All data points are from nonequilibrium MD simulations (Sahu et al., 2017; Sahu and Zwolak, 2017) except for the smallest pore in bi- (dashed line) and tri-layer graphene (dotted line), which were estimated from free-energy barriers. Lines are a guide to the eye only.