Abstract
Female athymic nude rats (Rattus norvegicus; n = 45; age, 6 wk) were used in an IACUC-approved protocol to investigate mechanisms and potential treatments associated with brain, spine, and spinal cord metastases from triple negative breast cancer. The analgesic plan included the use of buprenorphine SR LAB (0.6 mg/kg; 0.11 mL/rat) subcutaneously and an oral NSAID delivered via the water. Thirty-seven rats reached the experimental end point at 3 mo after xenotransplantation and were euthanized for tissue harvest. Grossly, all 37 rats had nodules in the subcutis over the shoulders; these were identified as small, cystic structures (diameter, approximately 0.25 cm). The cysts and haired skin were submitted for LC-MS/MS (liquid chromatography-tandem mass spectrometry) and histopathology. Histologically, the cysts were lined by fibrous connective tissue mildly infiltrated by macrophages, lymphocytes, and plasma cells. Adjacent blood vessels were rimmed by a mild infiltrate of lymphocytes and plasma cells. The cysts contained variable accumulations of a light pink, proteinaceous fluid. The cause for the cysts could not be determined histologically; there was no evidence of neoplasia. LC-MS/MS analysis revealed that the cysts contained buprenorphine. We hypothesize that the lack of T cells and a cell-mediated immune response in these rats prevented the dissolution of the vehicle and absorption of the buprenorphine. The manufacturer provides a cautionary statement regarding the use of this formulation in nude mice due to skin reactions, but to our knowledge, this report is the first description of an apparent lack of absorption of the drug in immunodeficient animals.
Abbreviation: Bup-SR, sustained-release buprenorphine LAB formulation (1 mg/mL)
Buprenorphine is an opioid commonly used to provide analgesia in laboratory animals. The advent of sustained-release formulations has made this drug more attractive to use, because they provide sustained analgesia for as long as 72 h without gaps in pain relief and reduces the need to repeatedly handle the animal, thus reducing stress.2,4,5
One compound analgesic available for use in veterinary medicine is buprenorphine SR LAB (Bup-SR; concentration, 1 mg/mL), which is a patented injectable, sustained-release polymer system designed to release buprenorphine over a 72-h period.9 This product has previously been shown to be effective in rats,1,4,6,7 and various laboratory animal formularies recommend its use.3 Therefore, our institution often uses sustained-release formulations of buprenorphine to provide analgesia in numerous rodent and large animal species.
A recent IACUC-approved study at our institution using athymic nude rats to investigate mechanisms and potential treatments using radiation therapy and engineered mesenchymal stem cells to treat brain, spine, and spinal cord metastasis included the use of subcutaneous Bup-SR (0.6 mg/kg; 0.11 mL per rat) and oral ibuprofen (0.15 mg/kg) to provide multimodal, preemptive analgesia for surgery. Xenotransplantation of triple negative breast cancer was performed intra-cranially, and into the lumbar vertebral body (L5) and lumbar spinal cord via a ventral abdominal approach to expose the lumbar spine. Ibuprofen was placed in the water bottle 48 h prior to surgery and continued for 5 d afterward; Bup-SR was given immediately prior to surgery. At the time of euthanasia and tissue harvest 3 mo later, subcutaneous nodules at the site of Bup-SR injections between the scapulae were noted in all animals that reached the experimental endpoint (37 rats). Due to the presence of these nodules, gross photos were obtained, tissues collected, and additional histologic and biochemical analyses were completed. This case report describes the findings of our investigation.
Case Report
Female athymic nude rats (Rattus norvegicus; n = 45; age, 6 wk; Charles River Laboratories, Frederick, MD) were used in a study to induce, treat, and analyze metastatic tumors of the brain and spinal cord. Rats were socially housed in autoclaved, static microisolation cages with corncob bedding (9092-9097 Teklad corncob bedding, Envigo, Greenfield, IN) and enrichment on 12:12-h dark:light cycle at 20 to 25 °C and 30% to 70% relative humidity. Rats were fed commercial food (PicoLab Irradiated Rodent Diet 20-5053, Purina, St Louis, MO) without restriction and municipal water (filtered to 1 µm and UV-treated) through sipper-tube water bottles. Rats were free of Toolan H1 virus (rat parvovirus), Kilham rat virus, lymphocytic choriomeningitis virus, Mycoplasma pulmonis, Pneumocystis carinii, pneumonia virus of mice, rat coronavirus (sialodacryoadenitis virus), reovirus 3, rat minute virus, rat parvovirus, rat theiliovirus, and Sendi virus as well as fur mites and pinworms, according to health reports from the vendor and sentinel testing. The study was approved by the Mayo Clinic IACUC and was conducted in compliance with the institution's AAALAC accreditation and OLAW assurance.
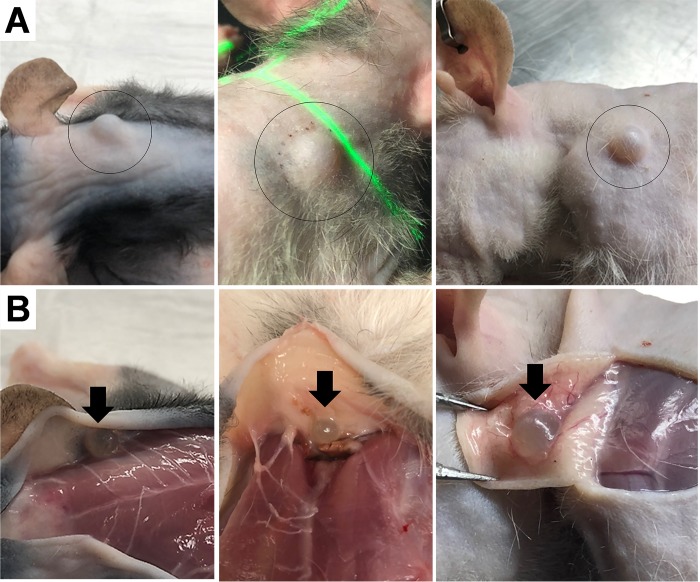
At the time of euthanasia, approximately 3 mo after xenotransplantation, subcutaneous nodules were detected grossly in the subcutis over the shoulders where Bup-SR (ZooPharm, Wildlife Pharmaceuticals, Windsor, CO) had been injected (Figure 1). These nodules were not found until necropsy and tissue harvest of the 37 rats that reached the experimental end point. The 8 rats that were removed from the study before reaching experimental end point were not necropsied so it is not known if they had these nodules in the subcutis. The lesions were grossly identified as small, cystic structures (diameter, approximately 0.25 cm). The cysts and haired skin were placed in neutral, buffered formalin and submitted to the Veterinary Medical Diagnostic Laboratory at Texas A&M University (College Station, TX) for LC–MS/MS and histopathology.
Figure 1.
(A) Gross morphology of 3 rats with dorsal subcutaneous lesions (black circles). (B) After euthanasia, the spheres were noted to be attached to the skin (black arrows).
A conference call among representatives of the compounding pharmacy and 2 of the coauthors (CDP and RSE) was made, to ascertain whether a formulation error occurred in the product that was received. The 2 lot numbers for the Bup-SR product used during the rat study were provided to the company representatives, but nothing in the quality-control documentation explained the findings in our rats.
Materials and Methods
To euthanize the animals at the end of the study and to fix the tissues, rats were deeply anesthetized by using intraperitoneal ketamine (30 to 50 mg/kg; VETone Zetamine CIII, 100 mg/mL, MWI, Boise, ID) and xylazine (5 to 10 mg/kg; VETone XylaMed 100 mg/mL, MWI) and intracardiac perfusion of 0.9% saline followed by 4% paraformaldehyde. In addition to the tissues harvested for the study, the tissue cysts were collected and submitted for analysis as described earlier.
Tissues were fixed in 10% neutral buffered formalin before sectioning and embedding in paraffin wax. Tissue sections (5 µm) were stained with hematoxylin and eosin for routine microscopic analysis.
Due to the dissolution of the sustained-release polymer vesicle by neutral buffered formalin, the formalin fluid in the container was analyzed by using LC-MS/MS according to a standard protocol.
Laboratory analysis.
The nodules and formalin solution were analyzed (Texas A&M Veterinary Medical Diagnostic Laboratory) for the presence of buprenorphine. Chemicals and reagents included methyl tert-butyl ether, acetonitrile (LC-MS/MS grade), ammonium hydroxide (28% ammonia solution, VWR International, Radnor, PA), formic acid (LC-MS/MS grade; Fisher Scientific, Pittsburgh, PA), and buprenorphine (Cerilliant, Round Rock, TX).
Nodule preparation.
Nodules were prepared by adding approximately 2.5 mL of phosphate buffer (0.1 M, pH 7.4) and homogenizing in a blender. Next, 1.25 mL of an inhouse deproteination reagent (zinc sulfate, sulfosalicylic acid, methanol, and water) was added. The samples were homogenized again and then centrifuged for approximately 10 min at 700 x g.
Nodule content and formalin extraction.
Nodule content and formalin extraction began by placing 1 mL of the prepared supernatant or formalin solution into a screw-top tube. Then, 100 μL of ammonium hydroxide (50%) was added to each tube, followed by 5 mL of methyl tert-butyl ether. Tubes were capped, rotary extracted for approximately 10 min, and centrifuged for approximately 5 min at 448 x g. The bottom layer was removed by aspiration. The supernatant was then transferred to a second glass tube and evaporated to dryness under a stream of nitrogen at approximately 45 °C. The residue was reconstituted in 80 µL of acetonitrile (5%) and transferred to an autosampler vial for LC-MS/MS analysis.
LC-MS/MS analysis.
LC-MS/MS was performed by using a triple quadrupole mass spectrometer with an electrospray ionization source (model 6400, Agilent, Santa Clara, CA) coupled to an Express C18 column (100 × 2.1 mm; inner diameter, 2.7 μm) with a C18 guard column (5 × 2.1 mm; inner diameter, 2.7 μm; Ascentis, St Louis, MO) maintained at 40 °C. Mobile phases A (water:formic acid, 100:0.1, v/v) and B (acetonitrile:formic acid, 100:0.1, v/v) were used to elute the analyte from the column. The following gradient elution was used at a flow rate of 0.5 mL/min: 0.0 to 0.1 min, 5% B; 0.1 to 2.0 min, 5% B to 50% B; 2.0 to 2.5 min, 50% B to 100% B; 2.5 to 3.0 min, 100% B; 3.0 to 3.1 min, 100% B to 5% B; and 3.1 to 5.5 min, 5% B. All MS/MS data were collected in positive ion mode through multiple reaction monitoring using a transition (m/z) of 468.3 to m/z 55.1, 101.1, and 84.1 for buprenorphine. The optimized parameter settings for electrospray ionization were: capillary voltage, 4.0 kV; gas temperature, 350 °C; gas flow, 10 L/min; and nebulizer, 50 psi. The injection volume was 10 μL for each sample.
Results
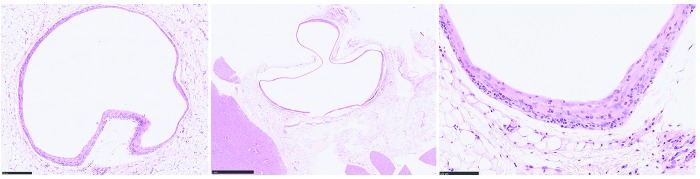
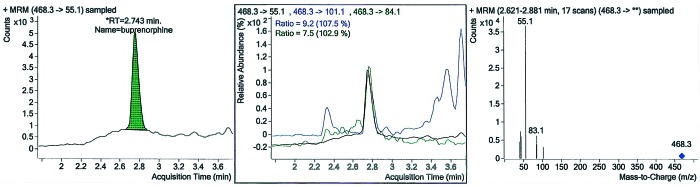
Histologically, cysts were lined by fibrous connective tissue entrapping mild infiltrates of macrophages, lymphocytes, and plasma cells. Adjacent blood vessels were rimmed by a mild infiltrate of lymphocytes and plasma cells. The cysts contained variable accumulations of a light pink, proteinaceous fluid (Figure 2). The cause for the cysts was not evident histologically; there was no evidence of neoplasia. LC-MS/MS analysis revealed that the cysts contained buprenorphine (Figure 3).
Figure 2.
Subcutaneous tissue from a rat with a subcutaneous nodule at 3 mo after injection. Bar = 250 µm. A fibrous connective band outlines where the polymer vesicle was present, 1 mm. Note the mild infiltrates of macrophages, lymphocytes, and plasma cells within the fibrous connective tissue band. Hematoxylin and eosin stain 100 µm.
Figure 3.
(A) The chromatogram of the formalin solution displays a buprenorphine peak at retention time (RT) 2.743 min. (B) Ion ratios of the formalin solution. (C) Chromatographic spectrum of the formalin solution.
Discussion
Bup-SR is a commonly used formulation for rodent analgesia. Except for anecdotal reports of skin reactions in athymic nude mice, no other detrimental side effects have been documented in the current literature. However, in a recent study that used this formulation in 45 athymic nude rats, we discovered that, in the 37 rats that reached the experimental end point, the sustained-release polymer system designed to release buprenorphine over a 72-h period was still intact, and the buprenorphine did not appear to have been released and absorbed. The remaining 8 rats used in the study either died (3 rats) or were euthanized (5 rats) after they developed hindlimb paralysis; these 8 rats were not necropsied. Although this formulation has been used in numerous rodent and large animal species at our institution, this study represents the first time that we have observed failure of the polymer vesicles to dissolve and release the buprenorphine.
The 37 rats that reached the experimental end point were unable to break down the polymer vesicle to release the buprenorphine for absorption (Figure 1). Histologically, it appeared that the rats had walled off the polymer with fibrous connective tissue that was mildly infiltrated by predominantly mononuclear inflammatory cells; this response created a dead-space artifact in the subcutaneous tissue (Figure 2). Whether any buprenorphine was released from the intact vesicle at the time of injection or over the 3-mo postoperative period is unknown, given that blood levels of buprenorphine were not measured. In another study, terminal blood samples collected through cardiocentesis of Sprague–Dawley rats that received 1.2 mg/kg Bup-SR (1 mg/mL) subcutaneously at 1, 2, 3, and 4 d after initial administration showed that the plasma concentration of buprenorphine was highest on day 2 (1.2 ± 0.3 ng/mL) but did not differ significantly from values on days 1, 3, and 4.6 This finding supported a previous finding that plasma concentrations of buprenorphine remained above 1 ng/mL for 72 h after an initial single dose of SR-Bup (1.2 mg/kg SC) in male Sprague–Dawley rats.4 To our knowledge, no one has evaluated buprenorphine blood levels after using a sustained-release product in immunodeficient rodents; this merits further investigation. After consultation with a Professor of Immunology (Mayo Clinic College of Medicine), we hypothesize that the lack of T-cells and a cell-mediated immune response in these rats prevented the dissolution of the polymer vehicle and absorption of the buprenorphine, but this notion requires further investigation in other immunodeficient rodent models. In addition, one publication that defined terminology and described the biodegradation of biorelated polymers stated that degradation of such a polymeric item was due to cell-mediated phenomena.8
LC-MS/MS clearly showed that the formalin fluid contained buprenorphine, which had been released from the dissolved polymer during tissue fixation in the neutral buffered formalin (Figure 3). Our method did not measure the amount of buprenorphine in the solution, but the gross appearance of the vesicle (Figure 1) suggested that considerable buprenorphine remained in the vesicle.
Due to concerns regarding the inability of immunodeficient rodents to break down the polymer vehicle of Bup-SR, our institution has discontinued the use of sustained-release formulations of analgesics in immunodeficient rodents until further studies can be done. Future studies will examine plasma concentration levels of buprenorphine and other analgesics that use a sustained-release polymer system in immunodeficient rodent models. This investigation will enable us to understand whether analgesic drugs are absorbed by immunodeficient rodents from the polymer system.
Acknowledgments
Special thanks to Dr Keith Knutson (Professor of Immunology, Department of Immunology, Mayo Clinic, Jacksonville, F), for his review and input on our initial abstract. This information was previously presented as an American College of Veterinary Pathology Late-Breaking Abstract and Poster at the 2018 ACVP and ASVCP Concurrent Annual Meeting, November 3–7, Marriott Wardman Park Hotel, Washington, DC.
References
- 1.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 2.DeTolla L, Sanchez R, Khan E, Tyler B, Guarnieri M. 2014. Subcutaneous implants of buprenorphine-cholesterol-triglyceride powder in mice. J Vet Med 2014:1–8. 10.1155/2014/365673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flecknell PA. 2016. Analgesia and postoperative care. p 182–183. Chapter 4. In: Flecknell PA, Laboratory animal anaesthesia, 4th ed Waltham (MA): Elsevier. [Google Scholar]
- 4.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 5.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343. 10.1038/laban.152. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RA. 2016. Voluntary running-wheel activity, arterial blood gases, and thermal antinociception in rats after 3 buprenorphine formulations. J Am Assoc Lab Anim Sci 55:306–311. [PMC free article] [PubMed] [Google Scholar]
- 7.Seymour TL, Adams SC, Felt SA, Jampachaisri K, Yeomans DC, Pacharinsak C. 2016. Postoperative analgesia due to sustained-release buprenorphine, sustained-release meloxicam, and carprofen gel in a model of incision al pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 55:300–305. [PMC free article] [PubMed] [Google Scholar]
- 8.Vert M, Doi Y, Hellwich K-H, Hess M, Hodge P, Kubisa P, Rinaudo M, Schué F. 2012. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem 84:377–410. 10.1351/PAC-REC-10-12-04. [DOI] [Google Scholar]
- 9.ZooPharm. [Internet]. 2013. Buprenorphine SR injectable. [Cited 11 January 2019]. Available at: https://wildpharm.com/media/djcatalog/BupSR-LAB_InfoSheet_2014.pdf