Fig. 2. Dual-labeled RIG-I reports 2CARD ejection by change in FRET.
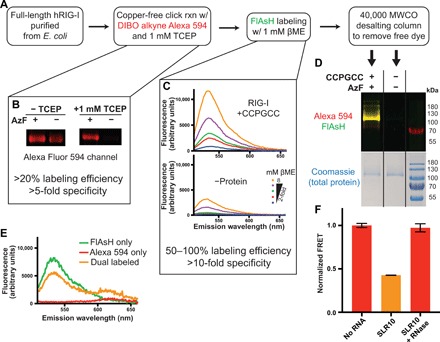
(A) A schematic representation of the process used to dual-label RIG-I with donor and acceptor fluorophores. Critical features of this protocol are highlighted. (B) One micromolar TCEP is required to reduce nonspecific labeling of RIG-I by DIBO alkyne Alexa Fluor 594. Labeled protein was analyzed by SDS–polyacrylamide gel electrophoresis (PAGE), followed by fluorescent imaging (excitation/emission: 532 nm/LP575). In the absence of TCEP, negative control protein lacking AzF (−AzF) is nonspecifically labeled. (C) βME is required for efficient FlAsH labeling (top), but large amounts of βME cause nonspecific fluorescence by free FlAsH (bottom). One micromolar βME was identified as an ideal concentration that is sufficient for labeling without causing background fluorescence. Fluorescent emission spectra were measured after FlAsH excitation at 479 nm. (D) The optimized protocol produces specifically dual-labeled RIG-I. Tagged and untagged proteins were both used in the dual-labeling protocol and analyzed by fluorescent imaging of an SDS-PAGE gel (Alexa Fluor excitation/emission: 532 nm/LP575; FlAsH excitation/emission: 473 nm/530DF20). Only tagged protein was covalently modified, demonstrating the specificity of labeling. (E) Dual-labeled protein exhibits FRET. Fluorescent emission spectra were measured after FlAsH excitation at 479 nm, demonstrating acceptor emission at ~615 nm, significantly above bleed-through fluorescence from direct excitation/emission of individual fluorophores. (F) RNA causes a decrease in FRET corresponding to an ejection of the 2CARD domain. Treatment with benzonase digests the RNA and resets the 2CARD domain. Error bars correspond to the SEM across replicate samples.
