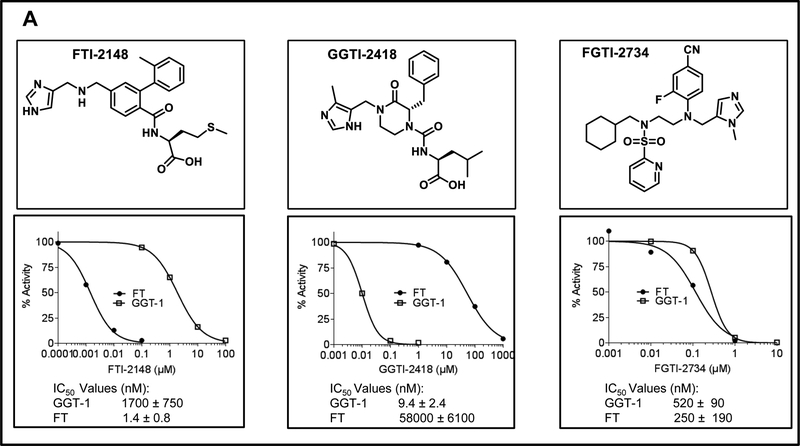
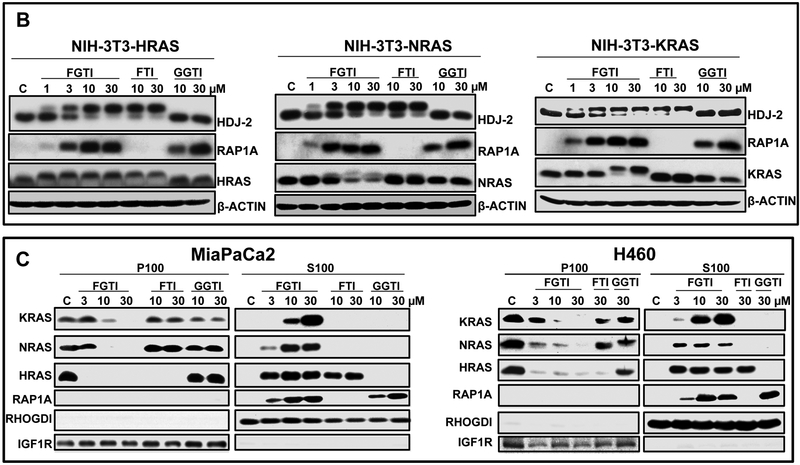
Figure 1. FGTI-2734, but not FTI-2148 or GGTI-2418, inhibits both protein prenylation and membrane localization of KRAS and NRAS.
A, Chemical structures and in vitro potency and selectivity of FTI-2148, GGTI-2418, and FGTI-2734. B, KRAS HRAS, and NRAS-transformed NIH3T3 cells were treated with vehicle (DMSO) or various concentrations of FGTI-2734, FTI-2148, and GGTI-2418 and processed for Western blotting with antibodies to KRAS, HRAS, NRAS, HDJ2, and RAP1A. Unprenylated bands of HDJ2, KRAS, HRAS, and NRAS show slower migration compared with fully prenylated HDJ2, KRAS, HRAS, and NRAS. RAP1A antibody can detect only unprenylated RAP1A. C, Human pancreatic cancer MiaPaCa2 and human lung cancer H460 cells were treated with vehicle (DMSO) or various concentrations of FGTI-2734, FTI-2148, and GGTI-2418, processed for membrane (P100) and cytosolic (S100) fractionation, and analyzed with Western blot using antibodies to KRAS, HRAS NRAS, and RAP1A. RHOGDI and IGF1R antibodies were used for internal controls for cytosolic and membrane fractions, respectively.