Fig. 1.
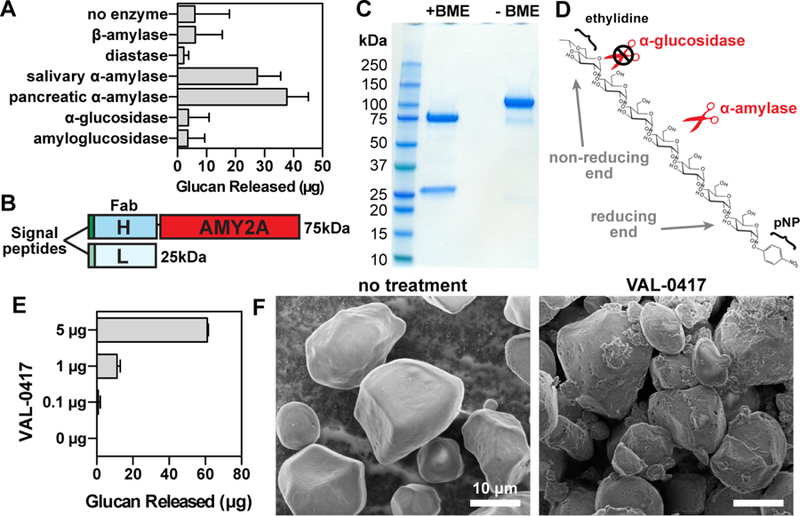
The antibody-enzyme fusion VAL-0417 degrades starch, a proxy for LBs. (A) Degradation of starch by a panel of amylases. 1 mg of starch was incubated with 5 µg of amylase overnight with constant agitation, then soluble and insoluble fractions were separated by centrifugation. Degradation product in the soluble fractions was quantified by measuring glucose equivalents (i.e. glucan). Reactions were performed in triplicate and data shown are means ± SD. (B) Schematic representation of VAL-0417. The gene encoding the human IgG1 Fab heavy chain fragment (H) was fused to AMY2A, encoding human pancreatic α-amylase, and coexpressed with the gene encoding the human light chain (L) in HEK293–6E cells. Heavy and light chain signal peptides were included to facilitate proper folding and assembly of the Fab fragment. The predicted molecular weight of each polypeptide is shown. (C) Purity of VAL-0417 assessed by reducing (+BME) and nonreducing (-BME) SDS-PAGE. (D) Chemical structure of the α-amylase-specific substrate E-G7-pNP. Reducing (inner) and non-reducing (outer) ends of the substrate are displayed. (E) Degradation of 1 mg starch after a 2-hour incubation with increasing amounts of VAL-0417, expressed as glucan released in the soluble fraction. Mean ±SD of triplicate reactions are shown. (F) Scanning electron micrographs of starch granules in the absence of treatment and after overnight treatment with VAL-0417. Samples were visualized at 2kV by an FE Quanta 250 scanning electron microscope. See also Figure S1.
