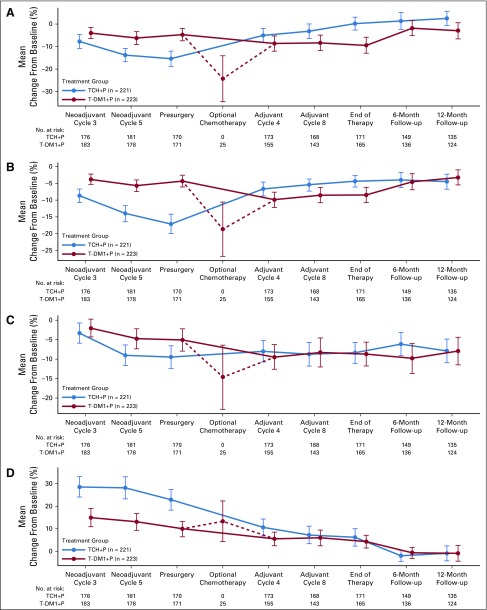
FIG 3.
Patient-reported outcomes during the overall study period for patients in the intention-to-treat population. Graphs depict mean change from baseline in European Organization for Research and Treatment of Cancer Quality-of-Life Questionnaire−C30 for domains of (A) global health status, (B) physical functioning, (C) cognitive functioning, and (D) diarrhea. Baseline is defined as neoadjuvant cycle 1, day 1. TCH+P, docetaxel, carboplatin, and trastuzumab plus pertuzumab; T-DM1+P, trastuzumab emtansine plus pertuzumab.