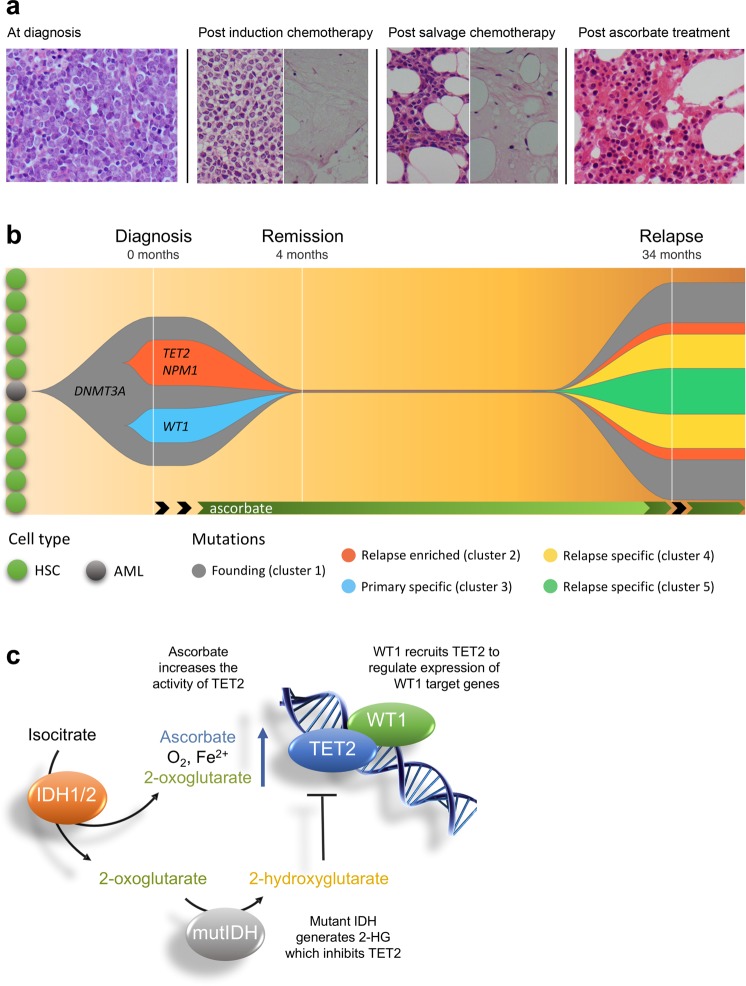
Fig. 1. Bone marrow biopsies, clonal evolution, and proposed mechanism for the effect of ascorbate in TET2 compromised AML.
a Bone marrow biopsies from the patient before and after treatment. At diagnosis, approximately 76% of total nucleated cells were blast cells. At 21 days following induction chemotherapy, trephine biopsy showed that normal hematopoietic tissue was almost completely replaced by blast cells (up to 80% of the total number of cells). In addition, there were areas of hypocellularity and necrosis. After salvage chemotherapy, there was persistent suppression of granulopoiesis with up to 65% blast cells and large areas of necrosis. Two months following ascorbate treatment, a bone marrow biopsy showed robust evidence of tri-lineage hematopoiesis and no blast cells, consistent with morphological remission. See Supplementary Fig. 2 for more images. b Clonal evolution of AML in this patient. The data used to generate this plot can be found in Supplementary Table 1. At least three major clusters were present in the diagnostic bone marrow. On the basis of variant allele frequency (VAF), the founding clone (cluster 1) contained somatic mutations in DNMT3A and eight other genes (STAT5B, EEF1A2, CLCN2, KCND3, ATP2C1, CFLAR, PALB2, FAT3). Subsequently, one subclone developed mutations in TET2, NPM1, and TAF2 (cluster 2) with a separate subclone developing mutations in WT1, ALDH16A1, and FAM8A1 (cluster 3). Whole exome sequencing post treatment with ascorbate did not detect any variants. At relapse, two of the three clones reemerged (clusters 1 and 2), with the addition of nine new mutations. The VAFs of the DEFA5 and MYC mutation place them within the TET2 subclone (cluster 4). Mutations in, ABCA1, PPM1E and TRIM29 (cluster 5) could either fall within, or outside, of the TET2 sublcone. The VAFs of RNF40, MTDH, DST and PBLD make them harder to place within the clonal structure and are not included here for clarity. This is potentially due to loss of heterozygosity which is supported by the fact that there are now VAFs greater than 50%. Black arrows indicate treatment with chemotherapy. HSC, hematopoietic stem cell. AML, acute myeloid leukemia stem cell. c Proposed mechanism for effect of ascorbate where mutations affect the IDH1/2-TET2-WT1 pathway. Loss of function mutations in WT1 or TET2 and gain of function mutations in IDH1 or IDH2, are early changes in the development of AML. These mutations are mutually exclusive in AML and all lead to decreased TET2 activity (see Supplementary Fig. 1) in 30–50% of AML. The TET enzymes are dependent on ascorbate for optimal activity, and providing additional ascorbate has been shown to increase the activity of TET2. Therefore, we propose that the benefit of ascorbate might extend to any mutation that affects the IDH1/2-TET2-WT1 pathway. MutIDH, Mutant IDH1 or IDH2. 2-HG, 2-hydroxyglutarate