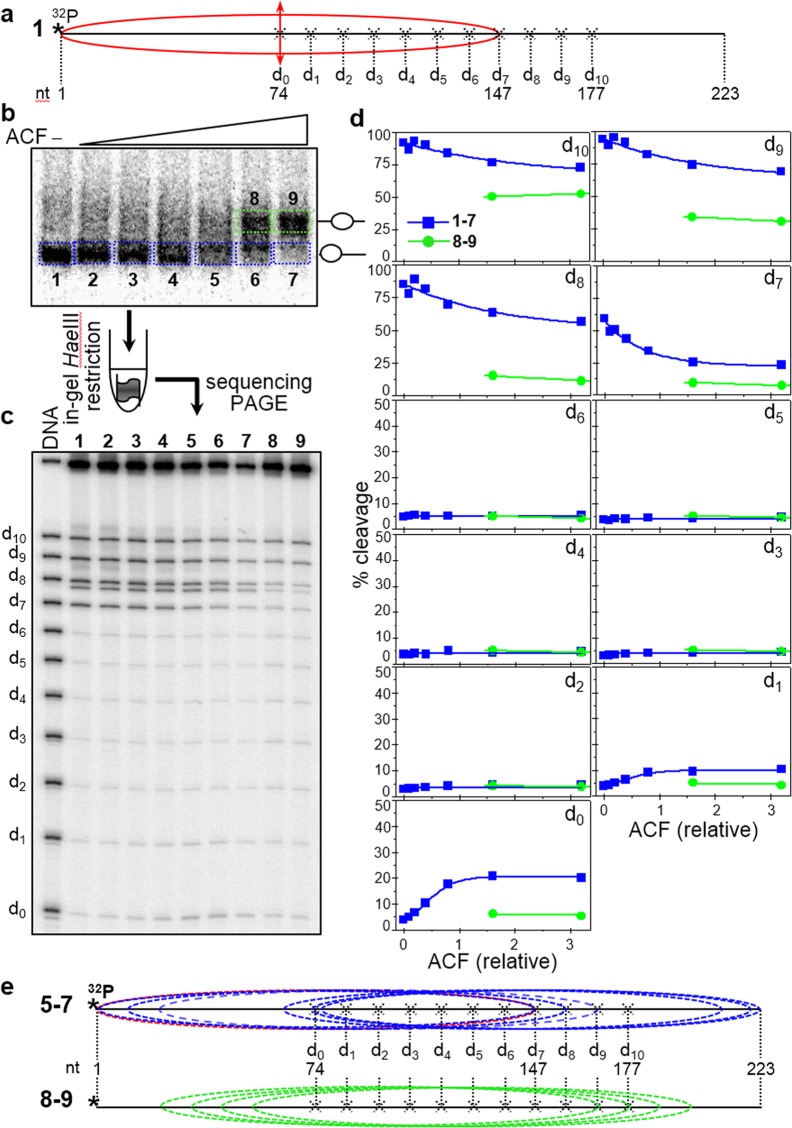
Figure 6.
Time course of HaeIII acessibility of ACF-remodeled nucleosomes in solution. (a) Schematics of the reconstituted nucleosomes. Eleven different 32P-end-labeled mutated 223 bp 601.2 DNA sequences were used to reconstitute end-positioned nucleosomes (each one of the sequences bears a unique HaeIII restriction site, designated d0 to d10, where the number indicates their position in helical turns from the dyad). (see schematics presented in (a) and in Fig. 2a). (b) An equimolar mixture of the 11 end-positioned nucleosomes was incubated with increasing amounts of ACF in the presence of ATP. The reaction mixtures were then separated on a 5% PAGE under native conditions. Then the lower bands (containing the particles with non-modified mobility) as well as the upper bands (containing the particles with modified, lower mobility) were excised from the gel and the particles were eluted from the gel slices. The eluted samples were digested with 4 units/μl of HaeIII for 5 minutes. The digested DNA was run on an 8% PAGE under denaturing conditions (c). (d) Quantification of the data presented in (c). After exposure of the dried gel, HaeIII digestion product bands for each restriction site the respective particle were quantified and expressed as a percentage of cut fraction in function of the amount of ACF used for remodeling; blues, fractions 1–7; green, fractions 8–9. (e) Upper panel, schematics of the Exo III mapped positions (relative to the DNA ends) of the nucleosomes within fractions 1–7 which exhibit non-modified (high) electrophoretic mobility; the position of the control particle, not treated with ACF is shown in red. Lower panel, same as the upper panel, but for the Exo III identified positions of the nucleosomes from fractions 8 and 9, showing modified, lower electrophoretic mobility.