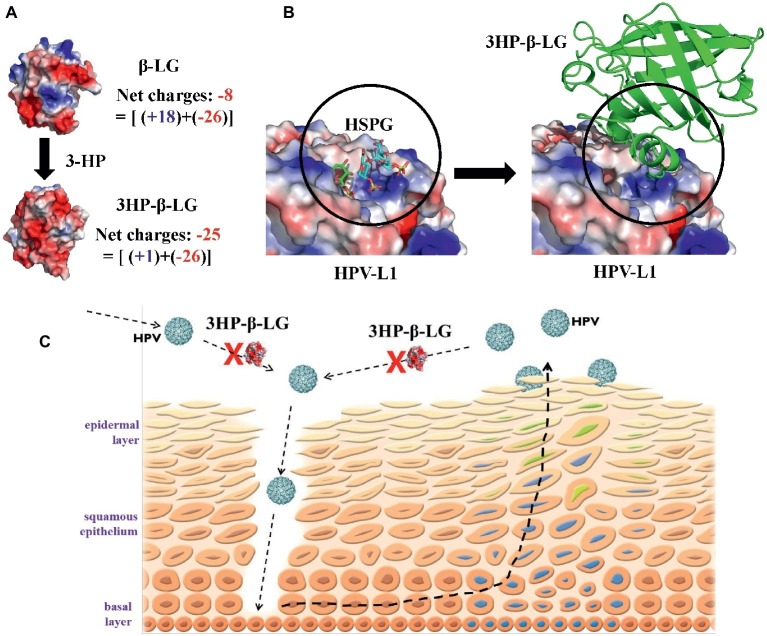
Figure 6.
Proposed mechanism by which 3HP-β-LG inhibits HPV infection in cervical mucosa. (A) The increased net negative charges on β-lactoglobulin after modification of the protein with 3-hydroxyphthalic anhydride. The structure of 3HP-β-LG was modeled by COOT (Emsley et al., 2010) using the crystal structure of β-LG (PDB entry: 1BEB). The structures were shown as an electrostatic surface by Pymol (Alexander et al., 2011). (B) Predicted interactions between HPV L1 pentamer and 3HP-β-LG. Crystal structure of HSPG-bound HPV L1 pentamer (PDB entry: 5W1O) was shown as electrostatic surface (Dasgupta et al., 2011), and the docking structure between HPV L1 and 3HP-β-LG was generated by AutoDock (Trott and Olson, 2010). (C) Schematic illustration showing how 3HP-β-LG inhibits HPV entry and infection. The newly invaded or newly released HPV particles access the basal layer through the mucosal lesion, or breaks, and bind to the HSPG receptor on the basement membrane, resulting in the entry of HPV into and replication in mucosal epithelial cells. 3HP-β-LG can bind with HPV L1 protein, through the electrostatic interaction between the negatively charged region in 3HP-β-LG and the positively charged region in L1 protein, to block the attachment of HPV to the HSPG receptor, resulting in inhibition of HPV infection in vaginal mucosa.