Abstract
Dl-3-N-butylphthalide (NBP) is approved in China for the treatment of ischemic stroke. Previous studies have shown that NBP promotes recovery after stroke via multiple mechanisms. However, the effect of NBP on vascular function and thrombosis remains unclear. Here, we aim to study the effect of NBP on vascular function using a rat model of transient middle cerebral artery occlusion (MCAO) and a state-of-the-art high-resolution synchrotron radiation angiography. Eighty SD rats underwent MCAO surgery. NBP (90 mg/kg) was administrated daily by gavage. Synchrotron radiation angiography was used to evaluate the cerebral vascular perfusion, vasoconstriction, and vasodilation in real-time. Neurological scores, brain infarction and atrophy were evaluated. Real-time PCR was used to assess the expression levels of thrombosis and vasoconstriction-related genes. Results revealed that NBP attenuated thrombosis after MCAO and reduced brain infarct and atrophy volume. NBP administrated at 1 and 4 h after MCAO prevented the vasoconstriction of the artery and maintained its diameter at normal level. Administrated at one week after surgery, NBP functioned as a vasodilator in rats after MCAO while displayed no vasodilating effect in sham group. Our results suggested that NBP attenuates brain injury via increasing the regional blood flow by reducing thrombosis and vasoconstriction.
Keywords: Cerebral ischemia, dl-3-N-butylphthalide, synchrotron radiation angiography, thrombosis, vasodilation
Introduction
Stroke is a leading cause of disability and death in the world. Many deficits occur after ischemic stroke in the brain, involving inflammation, reperfusion injury, and neurological dysfunction.1,2 Currently, the most effective strategies are thrombolysis and endovascular interventional in the early stage after ischemic stroke.3 The two methods both aimed at restoring the blood perfusion in the ischemic area as quickly as possible. Cell death of neurons and other functional cells leads to motor and cognitive deficits. Studies have found that the tissue next to the ischemic core present in a “functional silent” state named penumbra, which is the region that is potentially salvable after ischemic injury.4 These region is at risk, but it still has the potential to recover if sufficient blood supply can be restored after ischemia.5 Whether or not the tissue in the penumbra could survive critically depends on the blood flow and vessel perfusion.6 Since there is no strategy to reverse the injury in the ischemic core, the penumbra is thought as a diagnostic and treatment target.7 Collateral circulation or other direct blood flow augmenting therapies in the penumbra had been shown to reduce neuronal damage and improve behavioral outcome.8
Renin-angiotensin system (RAS) plays a key role in regulating blood vessel contraction and blood pressure.9 The local RAS in the brain plays a role in regulating systemic blood pressure and the cerebral blood flow, and regulating the RAS may be an efficient strategy on vascular function.10 Within thrombosis system, downstream effector of arachidonate such as prostacyclin inhibits platelet aggregation, while thromboxane is the main cause of thrombosis in animals.10,11 Studies confirmed that targeting the RAS and thrombosis system may improve the prognosis of stroke.12–14
Dl-3-N-butylphthalide (NBP) is a small molecule drug used in ischemic stroke treatment in China.15 It was first extracted from celery seeds and show protective effects for ischemic stroke, attributing to its anti-inflammation, anti-apoptosis, and pro-angiogenesis properties.16,17 Generally, the protective effects of NBP are studied within a couple of days after disease onset. Study found that NBP can increase the regional blood flow after brain ischemia, suggesting that NBP may have beneficial effects toward rescuing the penumbra.18 NBP has been reported to reduce the activation of caspase-3 and caspase-9 and inhibit apoptosis after ischemic injury. A decrease of JNK and P38 activation has also been observed.19 An in-vitro study demonstrated that NBP protects the brain microvascular endothelial cell against oxygen glucose deprivation-induced injury via up-regulating bcl-2 expression.20 Another study found that NBP can reduce cerebral edema and down-regulate RhoA expression, a downstream effector of RAS that can regulate the contraction of smooth muscle cell.21 The protection of both endothelial cells and blood–brain barrier by NBP suggests that the NBP holds potential in rescuing vascular function after ischemic injury. Previous studies were focused on the therapeutic effect of NBP on different vascular disease, but few of them paid attention to the vascular system. It is meaningful to know the regulation of NBP on the brain artery for a wider application in various vascular diseases like the vascular dementia. And a better understanding of NBP would make it safer to combine NBP with other strategies to treat these vascular diseases.
In this study, we investigated the vasodilation and anti-thrombosis effect of NBP after ischemic brain injury using a rat model. We examined the effect of NBP on the RAS and prostacyclin system. The impact of NBP on overall recovery and on vascular biology was assessed both in the acute and subacute phase after ischemic stroke.
Materials and methods
Animals
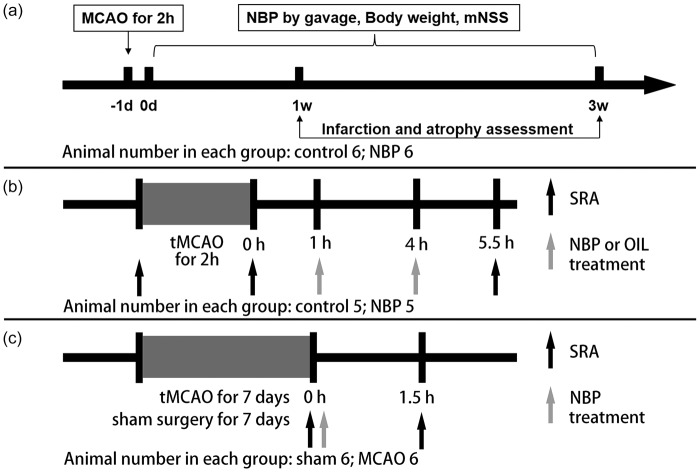
The ARRIVE guidelines were followed in the design and report of the current study. The experimental protocols were approved by the Institutional Animal Care and Use Committee of the School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China (Permission number: Bioethics 2012022). During the animal studies, guidelines of the regulation for the administration of affairs concerning experimental animals of China enacted in 1988 were followed. Rats were housed under standard laboratory conditions. A total of 130 adult male SD rats were used in this study, and all rats weighting 250–300 g aged eight weeks were randomly divided into each group. Twenty rats used to evaluate the long-time recovery of rats in two groups: (1) control group, (2) NBP administration group. Twenty rats used to assess the mRNA level three days after ischemia in three groups: (1) sham group, (2) control group, (3) NBP administration group. Fifty rats used to evaluate the thrombosis in artery and mRNA level seven days after ischemia in two groups: (1) control group, (2) NBP administration group. Twenty rats used to test the vasodilation effect of NBP in the acute phase of stroke in two groups: (1) control group, (2) NBP administration group. These rats all underwent ischemic surgery. Twenty rats used to assess the specific vasodilation effect of NBP in the subacute phase of stroke in two groups: (1) sham control group, (2) ischemic group, and rats in both groups received NBP administration. The experimental design is summarized in Figure 1.
A rat model of transient middle cerebral artery occlusion
The surgical procedure for the transient middle cerebral artery occlusion (tMCAO) has been previously described.22 Briefly, rats were anaesthetized by 5% chloral hydrate (400 mg/kg, intraperitoneally) and then positioned supinely on a heating pad (RWD Life Science, Shenzhen, China) to maintain the body temperature at 37℃. The skin at the neck was cut off, and artery on the left side was separated to be seen clearly under an operating microscope (Leica; Wetzlar, Germany). Then, a 4–0 nylon monofilament suture (Dermalon, 1756-31, Covidien, Mansfield, MA) coated with silicon was inserted from the external carotid stump into the internal carotid artery. The depth of suture insertion was approximately 18–20 mm until it blocked the opening of the middle cerebral artery. The success of occlusion was characterized by the reduction of cerebral blood flow to 20% of the baseline, which was verified by a laser Doppler flowmetry (Moor Lab, Instruments, Axminster, UK). The occlusion was allowed to continue for 2 h before the suture was gently withdrawn from the artery to allow reperfusion. The regional blood flow was assessed again by the laser Doppler flowmetry after the suture withdrawn, and an increase of cerebral blood flow to 70% of the baseline was characterized as successful reperfusion. The mortality of rats underwent tMCAO was 14.5% (16 of 110). The sham rats underwent the same surgery except the filament insertion. Body weight was assessed every day after the tMCAO surgery.
Neurobehavioral examination in rats
Neurological behavioral tests were carried out by another investigator blind to the experimental groups using a modified neurological severity score (mNSS) system.23 The mNSS ranges from 0 to 14, in which 0 represents normal behavior and 14 represents the highest degree of neurological deficiency. It is a comprehensive assessment of neurological function including motor, sensory, balance, and reflex tests. For the motor test, bend and torsion of limbs were observed by holding up the tail of the rat (0–3). The posture while walking on the floor was also assessed (0–3). For the balance test, the rats were placed on a beam. The neurological deficiency was assessed according to whether the rats could keep balance on the beam, limbs fall off the beam, and walk through the beam (0–6). For the sensory and reflex tests, pinna and corneal reflex were examined (0–2).
Synchrotron radiation angiography protocol
Imaging setup and procedures have been described previously.24 The X-ray energy was 33.3 KeV and the beam current was 250 mA, and the distance between the CCD detector and the animals was 65 cm. Animals were anaesthetized by 5% chloral hydrate (400 mg/kg, intraperitoneally) during the imaging process. A PE-10 tube was inserted into the common carotid artery (CCA) for contrast agent administration. An iohexol contrast agent (Ipamiro, shanghai, China) was injected into the CCA as a bolus (180 µl at 100 µl/s) using an automated micro-syringe pump (LSP01-1A, Longer; Baoding, China). An X-ray complementary metal oxide semiconductor (pixel size of 6.5 × 6.5 µm, frame frequency of 50 Hz, Hamamatsu Ltd, Hamamatsu City, Japan) was used to record the high-resolution real-time angiographic images. Contrast agent was administrated through CCA by a bolus injection (180 µl within 2 s) in this study.
Drug administration and experimental design
NBP (purity > 96%) was obtained from Shijiazhuang Pharmaceutical Group Co. Ltd (Shijiazhuang, China) and diluted in vegetable oil. The long-time pharmacology assessment experiment included two groups: the tMCAO with control group, and the tMCAO with NBP group. All animals in each group received NBP (90 mg/kg) or equal volume of vegetable oil by gavage once a day until the rats were sacrificed. Then the tissue from ischemic hemisphere was obtained by a same method for the rest of the experiment. The short-time cerebral vascular pharmacology assessment experiment included two groups: the tMCAO rats with control group, and the tMCAO rats with NBP group. Briefly, all rats in the two groups underwent SRA for three times: before MCAO surgery, right after reperfusion, 1.5 h after oil or NBP second administration. The oil and NBP administration (90 mg/kg) were given after 1 h and 4 h after reperfusion. The NBP-specific vasodilation effect experiment included two groups: the sham rats, and the rats that were seven days after tMCAO surgery. All rats underwent SRA for two times: before NBP treatment, and 1.5 h after NBP administration. The NBP administration (90 mg/kg) was given right after the first SRA.
SRA image processing and assessment of vessel internal diameter
All the images of SRA were obtained by the time-depended DSA method described previously.24 Briefly, the first image of each animal was set as the baseline, and the baseline was subtracted from the rest of images to remove the absorbance of tissues and bones. Measurements of vessel internal diameter of the MCA at the lenticulostriate artery bifurcation were carried out by two staffs who are blind to the sample history using image analysis software (ImageJ, NIH, Bethesda, MD). Vessel internal diameter represents the average of at least three independent measurements.
Infarct and atrophy volume measurement
All the brains were transcardially perfused with saline and fixed by 4% paraformaldehyde before being removed to freeze in −80℃ isopentane. A series of 20-µm-thick coronal sections were cut and mounted on slides, with an interval of 200 µm between each section, a total of 15–39 frozen sections from each rat were stained with Cresyl violet (sigma) and delineated by ImageJ. The samples were identified by numbers and the measurements of infarct and atrophy volumes were carried out by a person who is blind to the treatment history of the samples. Infarction volume between two adjacent sections was then calculated with the formula , where hwas the distance between two adjacent sections and Sn and were the infarction areas of two adjacent sections. Atrophy was calculated by using contralateral volume minus the ipsilateral volume by the same method above. Both infarction and atrophy were presented as a reality volume and a percentage compared with the contralateral hemisphere.
RNA isolation and RT-qPCR
The RT-qPCR was performed in rats before tMCAO, three days after tMCAO, and seven days after tMCAO. All rats were sacrificed by injecting excess 10% chloral hydrate, and brain tissue slices on the ischemic hemisphere (2 mm) were obtained by a brain matrix. Total RNA was isolated by Trizol reagent (Invitrogen), and reverse transcription was performed using an SYBR Premix Ex Taq Kit (Takara, Dalian, China) according to the manufacturer’s protocol. PCR which was performed with primers for all genes is shown in Table 1.
Table 1.
Gene names, PCR primers and amplicons.
| Gene name | Forward and reverse primers | Amplicon |
|---|---|---|
| REN | F: 5′- AGATGTGGTAACTGTGGGTG-3′ | 81bp |
| R: 5′- AGCATGAAGGGTATCAGGG-3′ | ||
| AGT | F: 5′- CACGGACAGCACCCTATTTT-3′ | 101bp |
| R: 5′- TGTTGTCCACCCAGAACTCA-3′ | ||
| ACE1 | F: 5′- GACATGGAGACGACTTACAG-3′ | 80bp |
| R: 5′- ATTTGTCAGATCAGGCTCC-3′ | ||
| AGTR1 | F: 5′- CACCCGATCACCGATCAC -3′ | 110bp |
| R: 5′- CAGCCATTTTATACCAATCTCTCA -3′ | ||
| RHOA | F: 5′- AGGATTGGCGCTTTTGGGTA-3′ | 128bp |
| R: 5′- ATGAGGCACCCCGACTTTTT-3′ | ||
| ROCK | F: 5′-AAGGGCAAATGCGGGAGTTA-3′ | 210bp |
| R: 5′- GAGCCAACTGCTCAGACTCA-3′ | ||
| GAPDH | F: 5′- GATGGTGAAGGTCGGTGTGA-3′ | 164bp |
| R: 5′- TGAACTTGCCGTGGGTAGAG-3′ | ||
| PTGES | F: 5′- AGGCTCAGGGTGAAGCAAAT -3′ | 111bp |
| R: 5′- GGTGCGGTTTTTAGCGGTTG -3′ | ||
| PTGIS | F: 5′- ATTCTCTCTTCGACGCGGTG -3′ | 218bp |
| R: 5′- CATAGCTCTTCCCCAGGCAC -3′ | ||
| TBXAS1 | F: 5′- ACCCTGAGACCTTTGACCCT -3′ | 85bp |
| R: 5′- CCAGCTCCAAAAGGCAGGTA -3′ | ||
PCR: polymerase chain reaction.
Statistical analysis
Values are expressed as mean ± SD in the text and figures. The data from treated and control groups were analyzed by Student’s t-test analysis of variance with SPSS Statistics 20.0 software (IBM Corp., Armonk, NY). The 95% confidence intervals were set and value of p < 0.05 was considered to indicate statistical significance.
Results
NBP improved stroke prognosis compared to the vehicle control
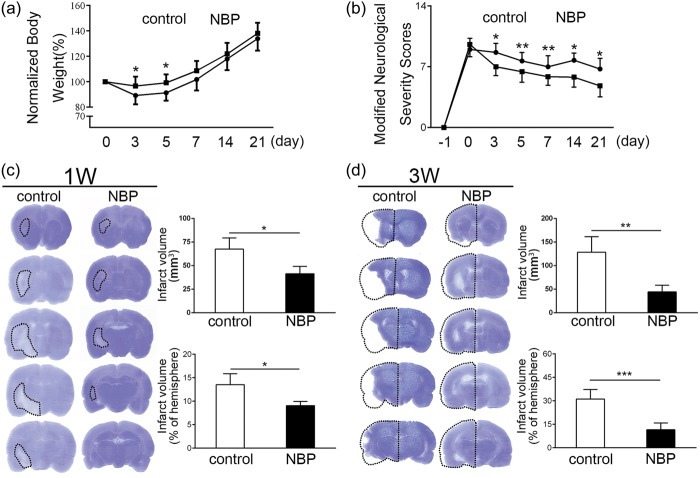
The body weight in the control group was reduced in the first five days after tMCAO. NBP treatment significantly ameliorated body weight loss compared to the control group in the first five days after tMCAO (Figure 2(a), p < 0.05, n = 6). However, no significant difference was observed in the following 17 days (Figure 2(a), p > 0.05, n = 6). The neurological behavior of the NBP group was significantly improved from 3 to 21 days after ischemia, assessed by mNSS (Figure 2(b), p < 0.05, n = 6). At one week after ischemia, the brain infarction volume of the NBP group (41.5 ± 7.9 mm3) was significantly smaller than that of the control group (67.6 ± 11.8 mm3, Figure 2(c), confidence interval: [5.7, 46.5], p < 0.05, n = 6). The brain atrophy volume of the NBP group (44.5 ± 14.1 mm3) was significantly smaller than that of the control group (128.5 ± 32.9 mm3) at three weeks after ischemia (Figure 2(d), confidence interval: [45.9, 122.2], p < 0.01, n = 6).
Figure 1.
Experimental design and schedules. (a) The long-term recovery assessment of rats. (b) The vasodilation effect of NBP in acute phase of tMCAO using SRA. (c)The vasodilation effect of NBP in sub-acute phase of tMCAO using SRA.
Figure 2.
The effect of NBP on the reduction of brain injury. (a) Body weight from the day 0 to the day 21 was all presented as normalized by comparing with the original body weight itself. In the early day 3 and day 5, normalized body weight in the NBP group was significantly higher than the control group. (b) After tMCAO model was established, modified neurological severity scores were tested until the rats were all sacrificed, in which the higher score represents a more serious deficit. The score in the NBP group was lower than the control group, which showed a significant neuroprotective effect of NBP on the ischemic rats. (c) The brain infarction was detected by Cresyl violet, which was circled by the dashed line, and the results were presented in volume and proportion. Both the infarction volume and proportion in the NBP group were significantly decreased than the control group. (d.)The brain atrophy was also detected by Cresyl violet, the dashed line represented the inversing contralateral that had no ischemia. Atrophy was calculated by using the volume of contralateral minus the volume of ipsilateral, and the results involved the atrophy volume and the proportion. Both the atrophy volume and proportion were significantly decreased than the control group. *p < 0.05, **p < 0.01, ***p < 0.001.
NBP regulated the gene expression of RAS and thrombosis system after tMCAO
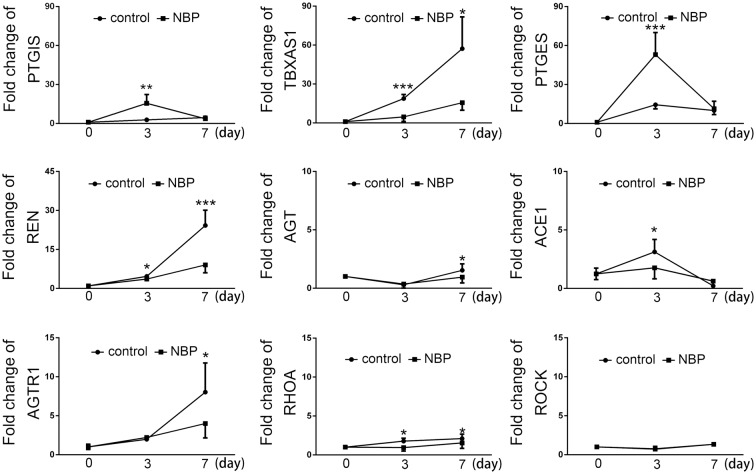
Previous results identified that NBP can increase cerebral perfusion of ischemic animal.18 To test the effect of NBP on blood vessel, qRT-PCR of key factors of vascular function and thrombosis was performed before surgery (0 day) and at three, seven days after tMCAO. Results showed that the genes of key factors in the thrombosis system, including prostacyclin synthase (PTGIS), thromboxane A2 synthase (TBXAS1), and prostaglandin E synthase (PTGES) are upregulated after tMCAO (Figure 3, p < 0.05, n = 6). The ratio of TBXAS1/PTGIS is an indicator of the risk of thrombosis. The upregulation of TBXAS1 after tMCAO caused TBXAS1/PTGIS ratio to increase, indicating a higher risk of thrombosis. NBP treatment promoted the expression of PTGES and PTGIS but attenuated the expression of TBXAS1 (Figure 3, p < 0.05, n = 6), and consequently reduced the ratio of TBXAS1/PTGIS and the risk of thrombosis.
Figure 3.
The effect of NBP on mRNA expression of thrombosis and vascular contraction system. Thrombosis-related factors PTGIS, TBXAS1, PTGES and vascular contraction-related factors REN, ANGIOTENSINOGEN, AGTR1, ACE1, RHOA and ROCK in the ischemic ipsilateral as detected by qRT-PCR. Bar graphs representing the statistical analysis, respectively. *p < 0.05, **p < 0.01, ***p < 0.001.
The qRT-PCR results also revealed that the genes of factors responsible for angiotensin biogenesis, including renin (REN), angiotensinogen (AGT), angiotensin converting enzyme 1 (ACE1) were upregulated at both three and seven days after tMCAO (Figure 3, p < 0.05, n = 6). Noticeably, NBP treatment significantly prohibited such upregulation (Figure 3, p < 0.05, n = 6). Downstream effector of angiotensin was also upregulated after tMCAO, including angiotensin II receptor type 1 (AGTR1) and RhoA at three and seven days after tMCAO, respectively (Figure 3, p < 0.05, n = 6).
NBP attenuated thrombosis after ischemic stroke
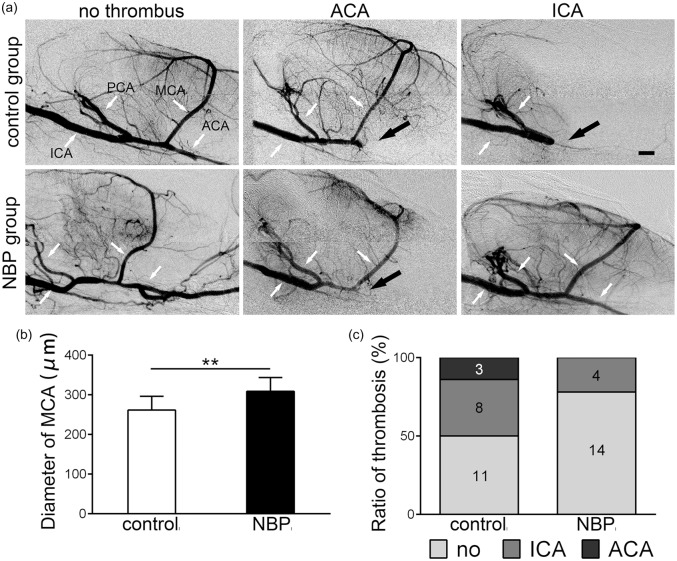
The SRA image of the cerebral vasculature of rats atone week after tMCAO revealed thrombus in the ipsilateral arteries of the circle of Willis in both the control and NBP group. The incidence of thrombosis in the ICA and ACA was counted in both control and NBP groups (Figure 4(a) and (c)). The incidence of thrombosis in the ACA and ICA was decreased in the NBP group compared to the control group. To evaluate the effect of NBP treatment on arterial diameter, we measured the diameter of MCA. The results revealed that the diameter of MCA in the NBP group (308.7 ± 34.8 µm) was significantly increased compared to the control group (264.7 ± 34.1 µm, Figure 4(b), confidence interval: [−27.7, −13.4], p < 0.05, n = 18).
Figure 4.
Continuous treatment with NBP for seven days attenuated the thrombosis in the ischemic artery. (a) SRA was performed seven days after ischemia. Three kinds of imaging results (no thrombus, ACA thrombus, ICA thrombus) of the SRA were showed in both control and NBP group. The white arrows indicated the unobstructed artery of the circles of Willis, and black arrows indicated the position of thrombus. (b) Diameter of middle cerebral artery, which was pointing in figure (a), was measured and continuous treatment of NBP for seven days significantly increased the diameter of MCA. (c) Numbers of all three kinds of thrombosis were presented. Ratio of ICA and ACA thrombus in NBP group was lower than the control group, and no ICA thrombus was observed in NBP group. *p < 0.05. Bar = 1 mm.
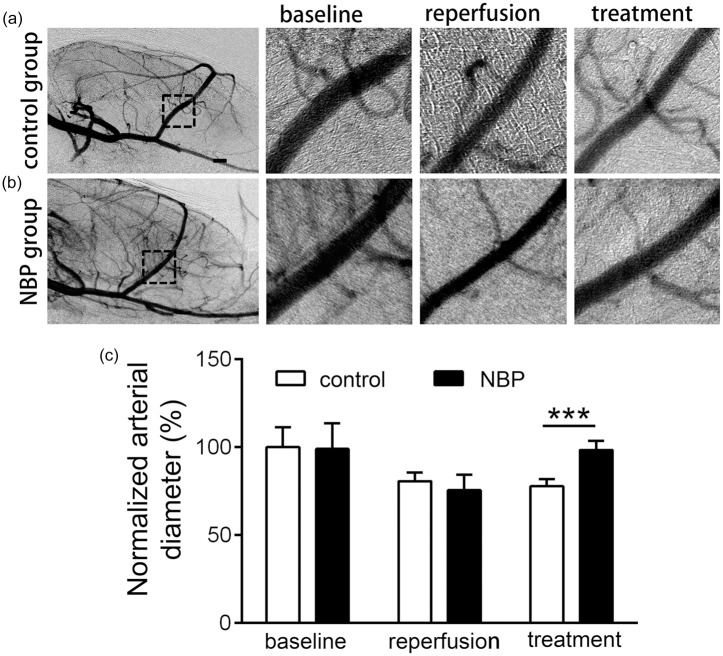
Vasodilation effects of NBP in the acute phase of stroke in rats
To assess the time window of the NBP’s vasodilation effect, we performed SRA at three time-points: before ischemia (labeled as “baseline” in Figure 5), immediately after reperfusion (labeled as “reperfusion” in Figure 5), and 5.5 h after reperfusion (labeled as “treatment” in Figure 5) (control or NBP treatment was administrated twice at 1 h and 3 h after reperfusion). The arterial diameter of MCA in the control (80.6 ± 4.8%) and NBP (75.7 ± 8.8%) groups both showed a decrease immediately after reperfusion, and the arterial diameter recovered to almost the original level after NBP treatment (98.4 ± 5.2%) while control had no effect on artery diameter (77.9 ± 4.1%, Figure 5(c), confidence interval: [−27.7, −13.4], p < 0.001, n = 5). These results support that NBP has vasodilation effect on MCA in the acute phase of stroke.
Figure 5.
Vasodilation effect of NBP on the MCA in the acute phase of tMCAO. (a) Rats in both control and NBP group underwent the SRA to obtain the vascular morphology and diameter of MCA was measured in the dashed outline (b) and (c). All rats in control and NBP groups were performed SRA three times, before tMCAO (baseline), immediately after reperfusion (reperfusion), and 5.5 h after reperfusion (control or NBP treatment was administrated at 1 h and 3 h after reperfusion, these groups are labeled as “treatment”). Vasoconstraction occurred after tMCAO in both groups, but only in NBP groups, the arterial diameter significantly increased than the reperfusion and recovered approximately to the level of baseline, which is significantly increased than the control group. ***p < 0.001. Bar = 1 mm.
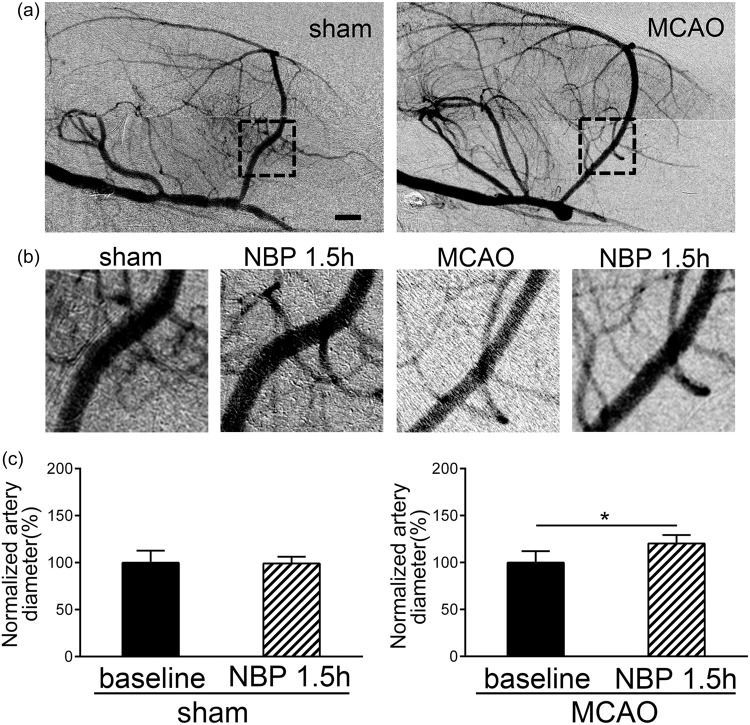
Vasodilation effect of NBP is specific to ischemic rats
We performed SRA on both the sham and the tMCAO rats at seven days after surgery, considered as the subacute phase. The baseline of MCA diameter in sham and ischemic group was acquired by the first SRA before NBP treatment, then rats in both groups received NBP. At 1.5 h after NBP treatment, the rats underwent the second SRA (Figure 6(a)). The diameter of MCA in both no MCAO and MCAO rats received NBP treatment was compared to the baseline level. The MCA of ischemic rats with NBP treatment (120.2 ± 9.0%) showed an angiectasis compared to the baseline (99.9 ± 12.2%, Figure 6(c), T = − 2.99, confidence interval: [−35.9, −4.6], p < 0.05, n = 6), while the diameter of MCA in sham rats (99.9 ± 12.8%) with NBP treatment (99.1 ± 4.8%) showed no significant changes (Figure 6(c), confidence interval: [−21.1, 22.9], p > 0.05, n = 6).
Figure 6.
Selective vasodilation effect of NBP on ischemic artery in subacute phase. (a) All rats were underwent SRA seven days after tMCAO surgery or no MCAO surgery to obtain the vascular morphology and diameter of MCA was measured in the dashed outline. (b) and (c) Rats in no MCAO and MCAO groups were underwent SRA for two times. The first SRA is to obtain the baseline of seven days after the tMCAO surgery or no MCAO surgery, and after NBP treatment for 1.5 h, the second SRA was to detect the vasodilation effect on both the normal and ischemic artery. NBP showed a vasodilation effect on the ischemic artery in the subacute phase of tMCAO, but not in the normal artery. *p < 0.05. Bar = 1 mm.
Discussion
In the present study, we demonstrated the anti-thrombosis and vasodilation effect of NBP in ischemic rats. NBP treatment reduced the brain infarct and atrophy volumes after cerebral ischemia and improved stroke prognosis. NBP attenuated the surgical-related thrombosis in tMCAO rats and functioned as a vasodilator after tMCAO from 3 h to seven days.
Stroke is often caused by the sudden occlusion of middle cerebral artery, and after the surgical or drug treatment, the ischemia brain is still in a critical situation, for the vascular function of artery and vein is out of balance.25 Both animal and clinical trials showed a higher risk of thrombosis in the arteries and micro-vessels after stroke, which means the blood vessel could be blocked again and lead to a secondary ischemic brain injury.22,26 The key point of clinical care is to help recover the blood supply of the ischemic region in time.
After ischemia, following the activation of the kinase system, great changes happen in many humoral regulation systems such as the RAS and the prostacyclin system. Once the imbalance of these systems happens, functional disorder occurs in the blood vessel and endothelium of the ischemic area. Regulating these kinase and their downstream molecules has been proven to be a good therapeutic strategy for preventing the secondary damage in the ischemic area.27 Our experiment examined the mRNA fold changes of these key factors after NBP administration in tMCAO rats. The upregulation of TBXAS1 with a lower increase of PTGIS, a higher ratio of TBXAS1/PTGIS, implies an increasing risk of thrombosis after tMCAO. But the increase of PTGIS, PTGES and decrease of TBXAS1, Renin, AGT, RhoA shows NBP administration rebalanced the mRNA level and may be effective on preventing the platelet aggregation and thrombosis.
Changes in the expression levels of the molecules in the two system imply that NBP improved the vascular function in tMCAO rats. The platelet aggregation in the artery after ischemia was an important inducer of thrombosis, and in many studies, the platelet inhibitors like Aspirin showed a long-term secondary prevention of serious vascular events in patients at high risk of vascular disease.28 Previous study also proved the thrombus after the tMCAO affects the reperfusion and leads to a worse neurological function outcome.22 NBP study in rats showed its anti-platelet effect on bleeding time and platelet activity, which imply us the NBP’s anti-platelet or anti-thrombosis function in vascular system.29 Our experiments showed that the novel evidence that NBP was able to attenuate the thrombosis in-vivo after tMCAO, suggesting that NBP not only inhibited the platelet aggregation in vitro, but also reduced the risk of thrombosis after tMCAO. Studies also demonstrated that NBP increased the blood perfusion of cerebral cortex in the ischemic animal, this could be the therapeutic effect of NBP on the ischemic core.18 When vasospasm of MCA occurs right after the tMCAO in few hours,30 the NBP treatment showed a vascular protective effect by reversing the vasoconstriction. Moreover, NBP treatment also increases MCA diameter in the subacute phase. Studies also demonstrated that pre-treatment of NBP protected the advanced glycation end products-induced injury in HUVECs in vitro.31 Pre-treatment of NBP also rescued the cerebral vascular disease like vascular dementia.32 We believed that pre-treatment of NBP may attenuate the damage of blood vessels. Although gene and protein expression were powerful evidence to demonstrate the function of NBP, it is out of focus of this study. And the molecule mechanism study will be performed in the future.
Less thrombus in the arteries represented a better blood supply to the ischemic region. In an untreated animal, most of the peri-infarct tissue, which was also called the penumbra, developed into necrosis tissue. That is why larger infarction area appears along with time after tMCAO. Apoptosis and necrosis factors in the ischemic core induced apoptosis and necrosis in the brain even near the infarct area, and regulating these pathways does attenuate the ischemia-reperfusion injury.33 The NBP treatment induced an increasing blood and oxygen to the ischemic region through the artery and micro-vessel, and that lead to the inhibition of apoptosis and auto-phage to prevent more neurons from cell death.
Many factors affect infarct volume in MCAO including animal species, gender, age, blood pressure, body temperature, occluding time course, and suture, etc. Suture is one important factor that impacts the infarction size. Key suture parameters include the silicone coating diameter and coating length. We carefully controlled these factors to control the size of the injury. In this synchrotron radiation angiography study, the animals were subjected to several injections of contrast agents. In order to reduce the mortality after surgery, we purposefully limited the injury by controlling the coating length of the suture. It should be noted that the occlusion to MCA was successful despite of this smaller infarction. The regional blood flow was reduced to 20% of the base line after suture insertion and returned to 70% of the baseline after reperfusion, monitored by a laser Doppler flow meter, supporting that this model is feasible for the study of ischemic injury and treatment. Our results demonstrated that NBP has vasodilation effect on major cerebral arteries in the acute and subacute stage of ischemic stroke and that NBP ameliorated ischemic injury and promoted the recovery after stroke in the subacute stage. NBP promoted perfusion and improved the prognosis after stroke. Such effect may be related to NBP-induced vasodilation and thrombosis inhibition effects. These results support that NBP is beneficial in both the acute and subacute phase after ischemic stroke.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (NSFC) projects 81522015 (YW), 81371305 (YW), 81771251 (GYY), 81471178 (GYY), and 81771244 (ZZ).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CQ was involved in research design, experimental performances, animal surgery, drafting the manuscript except neurobehavioral tests, and data analysis. PZ performed animal surgery. LW and MM participated in animal behavioral tests and data collecting. WL gave technical assistant. ZZ discussed the results and edited part of manuscript. GYY and YW are the corresponding authors; they took care of all aspects including research design, data analysis and manuscript preparation. All authors read and approve the final manuscript.
References
- 1.Kaur H, Prakash A, Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology 2013; 92: 324–334. [DOI] [PubMed] [Google Scholar]
- 2.Sharp FR, Lu A, Tang Y, et al. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab 2000; 20: 1011–1032. [DOI] [PubMed] [Google Scholar]
- 3.Medcalf RL, Davis SM. Plasminogen activation and thrombolysis for ischemic stroke. Int J Stroke 2012; 7: 419–425. [DOI] [PubMed] [Google Scholar]
- 4.Manning NW, Campbell BC, Oxley TJ, et al. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke 2014; 45: 640–644. [DOI] [PubMed] [Google Scholar]
- 5.Heiss WD. The concept of the penumbra: can it be translated to stroke management? Int J Stroke 2010; 5: 290–295. [DOI] [PubMed] [Google Scholar]
- 6.Fisher M. The ischemic penumbra: identification, evolution and treatment concepts. Cerebrovasc Dis 2004; 17(Suppl 1): 1–6. [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Donnan GA, Davis SM. Vessel occlusion, penumbra, and reperfusion - translating theory to practice. Front Neurol 2014; 5: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winship IR, Armitage GA, Ramakrishnan G, et al. Augmenting collateral blood flow during ischemic stroke via transient aortic occlusion. J Cereb Blood Flow Metab 2014; 34: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendoza A, Lazartigues E. The compensatory renin-angiotensin system in the central regulation of arterial pressure: new avenues and new challenges. Ther Adv Cardiovasc Dis 2015; 9: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen Dinh Cat A, Touyz RM. A new look at the renin-angiotensin system – focusing on the vascular system. Peptides 2011; 32: 2141–2150. [DOI] [PubMed] [Google Scholar]
- 11.Zhang XH, Zhou SY, Feng R, et al. Increased prostacyclin levels inhibit the aggregation and activation of platelets via the PI3K-AKT pathway in prolonged isolated thrombocytopenia after allogeneic hematopoietic stem cell transplantation. Thromb Res 2016; 139: 1–9. [DOI] [PubMed] [Google Scholar]
- 12.Fang W, Wei J, Han D, et al. MC-002 exhibits positive effects against platelets aggregation and endothelial dysfunction through thromboxane A2 inhibition. Thromb Res 2014; 133: 610–615. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy CA, Facey LJ, Widdop RE. The protective arms of the renin-angiontensin system in stroke. Curr Hypertens Rep 2014; 16: 440. [DOI] [PubMed] [Google Scholar]
- 14.Inaba S, Iwai M, Tomono Y, et al. Exaggeration of focal cerebral ischemia in transgenic mice carrying human Renin and human angiotensinogen genes. Stroke 2009; 40: 597–603. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Yun W, Zhang Q, et al. Mobilization of circulating endothelial progenitor cells by dl-3-n-butylphthalide in acute ischemic stroke patients. J Stroke Cerebrovasc Dis 2016; 25: 752–760. [DOI] [PubMed] [Google Scholar]
- 16.Wang HM, Zhang T, Huang JK, et al. 3-N-butylphthalide (NBP) attenuates the amyloid-beta-induced inflammatory responses in cultured astrocytes via the nuclear factor-kappaB signaling pathway. Cell Physiol Biochem 2013; 32: 235–242. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Lu L, Chan WM, et al. Effects of DL-3-n-butylphthalide on vascular dementia and angiogenesis. Neurochem Res 2012; 37: 911–919. [DOI] [PubMed] [Google Scholar]
- 18.Yan CH, Feng YP, Zhang JT. Effects of dl-3-n-butylphthalide on regional cerebral blood flow in right middle cerebral artery occlusion rats. Zhongguo Yao Li Xue Bao 1998; 19: 117–120. [PubMed] [Google Scholar]
- 19.Li J, Li Y, Ogle M, et al. DL-3-n-butylphthalide prevents neuronal cell death after focal cerebral ischemia in mice via the JNK pathway. Brain Res 2010; 1359: 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Li L, Huang R, et al. Hypoxia inducible factor-1alpha mediates protection of DL-3-n-butylphthalide in brain microvascular endothelial cells against oxygen glucose deprivation-induced injury. Neural Regenerat Res 2012; 7: 948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Wen Q, Wu Y, et al. The effect of butylphthalide on the brain edema, blood-brain barrier of rats after focal cerebral infarction and the expression of Rho A. Cell Biochem Biophys 2014; 69: 363–368. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Miao P, Wang J, et al. Surgery-related thrombosis critically affects the brain infarct volume in mice following transient middle cerebral artery occlusion. PLoS One 2013; 8: e75561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Li Y, Jiang L, et al. Macrophage depletion reduced brain injury following middle cerebral artery occlusion in mice. J Neuroinflamm 2016; 13: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu ZH, Jiang Z, Lin XJ, et al. Vessel dilation attenuates endothelial dysfunction following middle cerebral artery occlusion in hyperglycemic rats. CNS Neurosci Ther 2016; 22: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipolla MJ, Chan SL, Sweet J, et al. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke 2014; 45: 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanacker P, Cordier M, Janbieh J, et al. Floating arterial thrombus related stroke treated by intravenous thrombolysis. Cerebrovasc Dis 2014; 38: 117–120. [DOI] [PubMed] [Google Scholar]
- 27.Papademetriou V. Inhibition of the renin-angiotensin-aldosterone system to prevent ischemic and atherothrombotic events. Am Heart J 2009; 157: S24–S30. [DOI] [PubMed] [Google Scholar]
- 28.Sandercock P, Gubitz G, Foley P and Counsell C. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No.: CD000029. DOI: 10.1002/14651858.CD000029. [DOI] [PubMed]
- 29.Peng Y, Zeng X, Feng Y, et al. Antiplatelet and antithrombotic activity of L-3-n-butylphthalide in rats. J Cardiovasc Pharmacol 2004; 43: 876–881. [DOI] [PubMed] [Google Scholar]
- 30.Ahnstedt H, Mostajeran M, Blixt FW, et al. U0126 attenuates cerebral vasoconstriction and improves long-term neurologic outcome after stroke in female rats. J Cereb Blood Flow Metab 2015; 35: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CY, Zhao ZH, Chen ZT, et al. DL-3-n-butylphthalide protects endothelial cells against advanced glycation end product-induced injury by attenuating oxidative stress and inflammation responses. Exp Ther Med 2017; 14: 2241–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huai Y, Dong Y, Xu J, et al. L-3-n-butylphthalide protects against vascular dementia via activation of the Akt kinase pathway. Neural Regenerat Res 2013; 8: 1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu B, Ruan M, Liang T, et al. Tetramethylpyrazine phosphate and borneol combination therapy synergistically attenuated ischemia-reperfusion injury of the hypothalamus and striatum via regulation of apoptosis and autophagy in a rat model. Am J Transl Res 2017; 9: 4807–4820. [PMC free article] [PubMed] [Google Scholar]