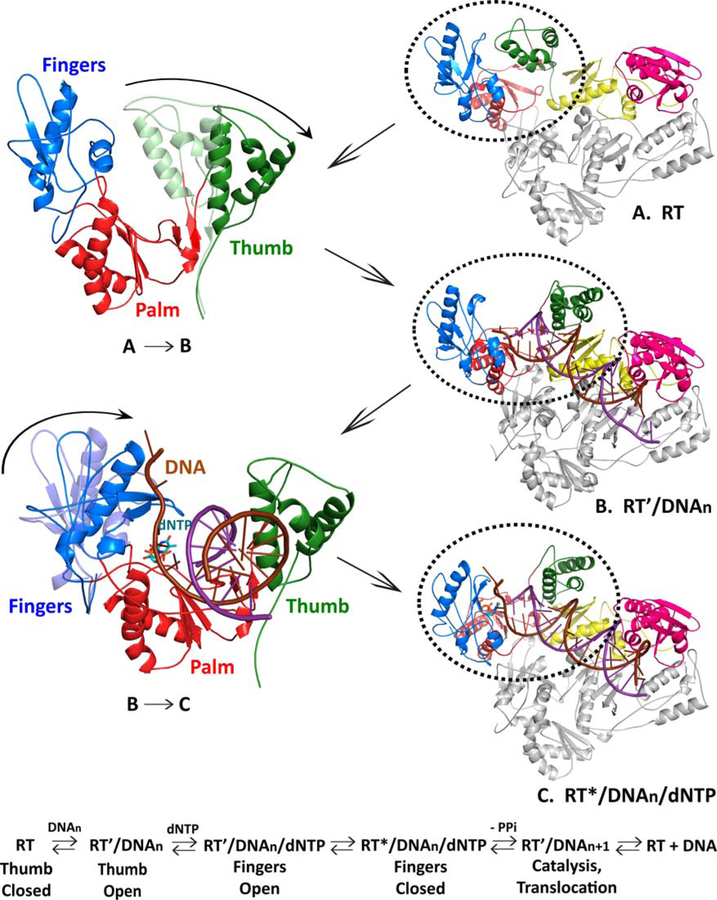
FIGURE 3.
Movement of HIV-1 RT during polymerization. The colors used for the various subunits, domains, and subdomains of RT, and for the two DNA strands, are as in Fig. 2. The p51 subunit is gray. The RNase H domain is shown in pink, and the four subdomains to the polymerase domain are as follows: fingers, blue; thumb, green; palm, red; and connection, yellow. The DNA template is dark red and the primer strand is purple. The incoming dNTP is light blue. The structural changes in RT can be correlated with specific steps in the binding of the substrates and the incorporation of the incoming dNTP. In unliganded RT, the fingers and thumb are closed (A). Before the dsDNA (or other nucleic acid substrate) can be bound, the thumb must move (A → B); this allows the dsDNA to be bound (B). This sets the stage for the binding of the incoming dNTP. When the incoming dNTP binds, the fingers close, which allows the dNTP to be incorporated, with the release of pyrophosphate (PPi). The incorporation of the dNTP temporarily leaves the end of the primer stand in the N or nucleoside triphosphate-binding site (see text). Translocation moves the nucleic acid by 1 bp, which allows the next dNTP to be bound and incorporated. doi:10.1128/microbiolspec.MDNA3–0027-2014.f3