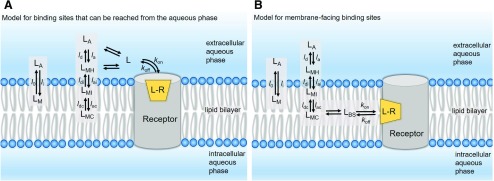
Models that account for membrane partitioning of lipophilic/amphiphilic ligands binding to solvent-exposed binding site (A) and transmembrane lipid-exposed binding site (B). The fundamental prerequisite for a drug (ligand) to elicit its biologic effect is the molecular recognition process in which the ligand molecule (
L) selectively binds to its target receptor (
R) to form a receptor-ligand complex (
LR). For a simple one-to-one reversible bimolecular reaction (eq. 1), the binding affinity of the receptor-ligand complex at equilibrium can be characterized by the dissociation constant,
Kd. The equilibrium dissociation constant (typically measured in moles per liter) is the ratio between the rates of the backward (
koff) and forward (
kon) reactions, which in turn, are directly proportional to the concentrations of free ligand, unoccupied receptor, and receptor-ligand complexes, respectively (eq. 2).
The association rate (forward reaction) is dependent on the
kon value as well as the ligand concentration in the target compartment. Typically, the endogenous ligands of GPCRs are polar and are assumed to reach their orthosteric binding sites directly from the extracellular aqueous compartment (
LA). The equations used to calculate the overall affinity constant and kinetic rates assume that the interacting molecules are homogeneously distributed and concentration of ligand available to bind target is equal to that in the bulk aqueous phase. However, it is important to recognize that highly lipophilic and/or amphiphilic ligands may partition into the surrounding membrane compartment driven by their equilibrium membrane partition coefficient,
Kmem and alter the observed pharmacology.
Kmem is the ratio between the equilibrium concentrations of ligand molecules in the membrane compartment (
cmem) and the aqueous phase (
caq);
Kmem can also be calculated from the transfer rates
li and
lo (see eq. 3; part A).
where li and lo are the membrane wash-in and washout rates of the ligand, respectively. In this scenario, the ligand concentration near the receptor is different from its concentration in the aqueous bulk. On the basis of their lipophilicity and amphiphilicity profiles and their ionization states, ligand molecules may reside at discrete locations within the membrane compartment: 1) at the surface, interacting with hydrated polar head groups (LMH); 2) at the interface between the polar head groups and the hydrophobic tails, interacting with both strata (LMI); or 3) at the hydrophobic lipid core (LMC). For compounds with high lipophilicity and/or amphiphilicity, the equilibrium concentration near the binding site can be affected by the magnitude of individual transfer rates (see part A) representing adsorption and desorption (subscripts “a” and “d” respectively) at the headgroup, interface, and core regions of the membrane.
For lipophilic and/or amphiphilic compounds binding to extracellular orthosteric sites, the contributions of membrane partitioning and interactions with various bilayer strata (and thus the local concentration) to the intrinsic
kon and
Kd values can be accounted for by the following equations (eqs. 4–6):
where
kon′ is the measured association rate constant. It is important to note that this relationship (eq. 4) is true only if the membrane equilibration time for the ligand is fast relative to its receptor association and dissociation rates.
Here, Kd′ and Kd represent the “observed” or “apparent” dissociation affinity constant (determined in bulk experimental studies) and “intrinsic” dissociation affinity constant of the ligand calculated after correcting for the membrane contribution, respectively. The most challenging scenario arises when the binding site is deeply embedded within the membrane and ligands can reach their binding sites only through lipid pathways (see part B). In such cases, the drug concentration near the binding site (LBS) is dependent on the location of the binding site within the membrane compartment relative to the drug’s preferred membrane location—for example, at the membrane core (LMC), interface (LMI) or hydrated headgroup (LMH)—as well as the diffusion coefficient of the ligand in question. It is important to recognize that Kmem-based adjustments may often underestimate the effect of the membrane partitioning of ligands, because the ligand concentration in the vicinity of the receptor may be different from the concentration in the bulk membrane owing to discrete localization effects. Also, the actual volume of the membrane occupied by the drug is substantially less than the total membrane volume.
In contrast to the association rate (kon), the dissociation rate (koff) is independent of the ligand concentration and depends solely on the specific molecular interactions of the ligands with the binding site residues. For ligands targeting membrane-facing binding sites, the participation of lipid molecules in the binding process (as one of the components of the binding site) needs different treatment, as these interactions would affect koff as well. The extent to which the membrane lipids are capable of engaging in the binding process can be appreciated by noting the significant membrane-exposed surface area of the ligands in the analyzed crystal structures (see Fig. 5; Table 1).