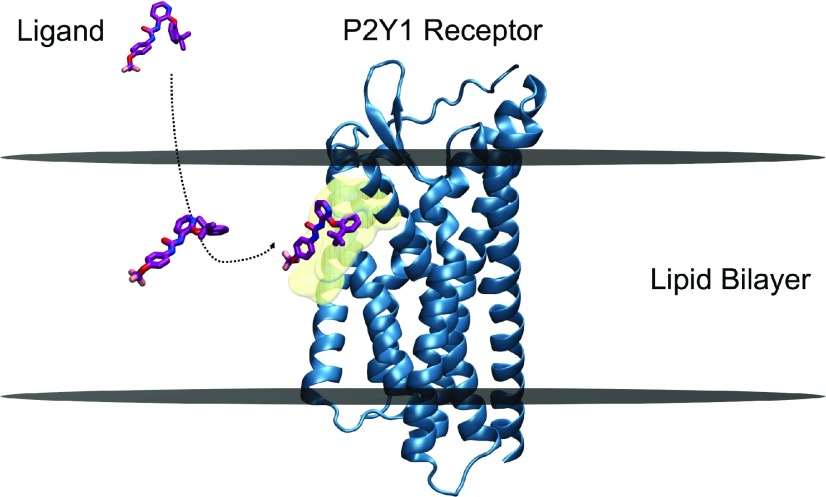
Fig. 3.
Membrane-facilitated ligand access and binding. BPTU, a small-molecule antagonist (negative allosteric modulator) partitions within the membrane first, and reaches the transmembrane extrahelical binding site of the P2Y1 receptor through a lipid pathway. The preferred orientation and conformation of BPTU within the POPC bilayer near the binding site, obtained by funnel metadynamics simulations, is strikingly similar to that of the bound ligand in the crystal structure, indicating the plausible role of membrane lipids in “pre-organizing” the ligand for receptor binding. BPTU and P2Y1 receptor molecules are represented in stick and secondary structures, respectively. The approximate positions of the upper and lower leaflets of a hypothetical lipid bilayer are marked by two horizontal lines perpendicular to the bilayer normal. The binding site residues are depicted as transparent surface models in the color yellow. Picture adapted from Yuan et al. (2018).