Figure 1. Randomization, Trial-Group Assignment, and Follow-up for Part 1 of the Trial.
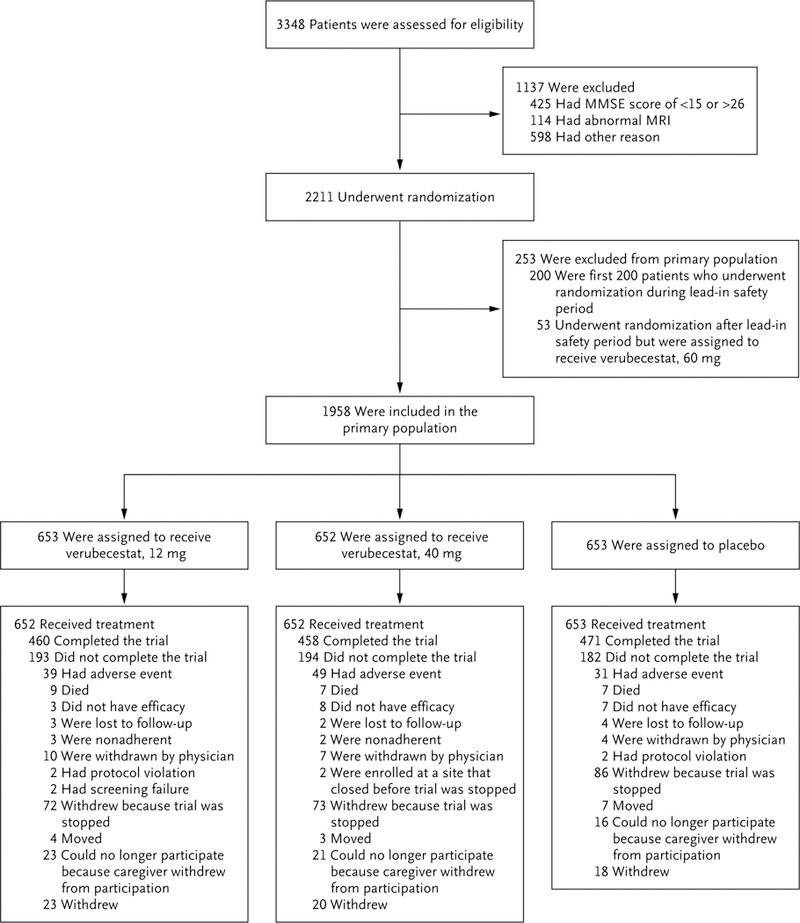
Part 1 of the trial was designed to include a phase 2 lead-in safety cohort component. In the phase 2 lead-in safety period, patients were randomly assigned to one of three dose levels of verubecestat (12 mg, 40 mg, or 60 mg) or placebo. The first planned interim analysis, which was conducted 3 months after the first 200 patients underwent randomization, informed the decision to progress to phase 3. The data from these first 200 patients were excluded from the primary efficacy and safety analyses. Randomization continued during the 3 months after the 200th patient was enrolled, during which time approximately 200 additional patients were randomly assigned to a trial group, including 53 patients who were assigned to receive a 60-mg dose of verubecestat and whose data were excluded from the primary analyses. Scores on the Mini–Mental State Examination (MMSE) range from 0 to 30, with lower scores indicating poorer cognitive performance. MRI denotes magnetic resonance imaging.
