Table 2:
Summary of Treatment Response and Survival: Results of Univariable Analysis
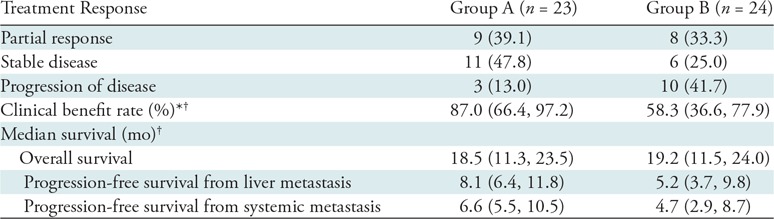
Note.—Unless otherwise specified, data are the number of participants, with percentages in parentheses. Group A includes treatment-naïve participants (no prior liver-directed and systemic therapies) and group B includes participants who experienced hepatic tumor progression following immunoembolization.
* Rate was calculated as follows: complete response + partial response + stable disease.
† Data in parentheses are 95% confidence intervals.
