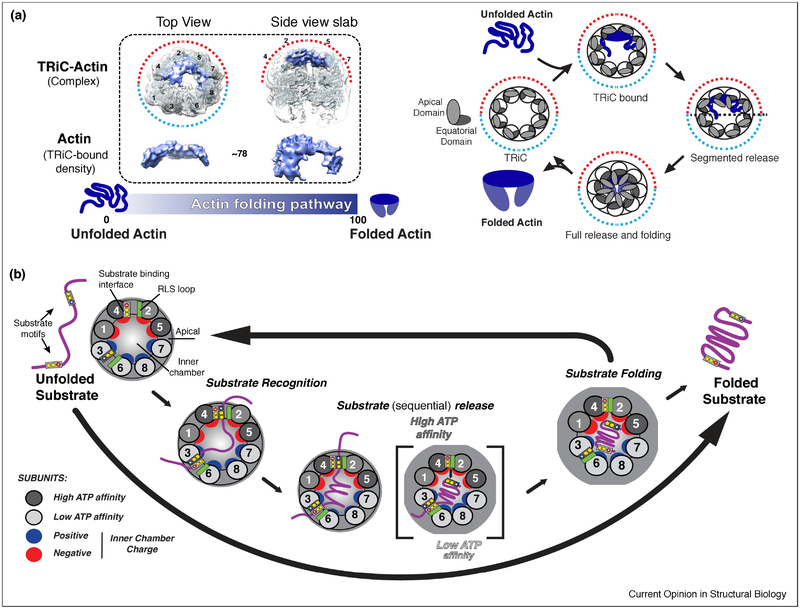
Figure 4: Role of TRiC subunit diversity in substrate folding and release.
(A) TRiC-Actin folding cycle adapted from (Balchin et al., 2018). Denatured Actin binds to apical domains on the high ATP affinity side of TRiC in a largely structured conformation. ATP binding and hydrolysis drives the sequential release of substrate into chamber allowing folding to occur; opening of the lid allows release of Actin into solution. (B) Overall model of TRiC mediated folding. Unique H11/PL binding interfaces allow TRiC to bind substrate (Purple) in a subunit specific conformation during initial recognition. The asymmetry of the ATPase cycle (high to low affinity; dark to light grey), initiates a sequential closure of the TRiC complex. Proximal interactions of the RLS loop (green) and the H11/PL binding interface facilitates sequential substrate release into the folding chamber. In the chamber, substrate is subjected to an asymmetric distribution of charges with the surface of one hemisphere positively (blue), and the other negatively (red) charged.