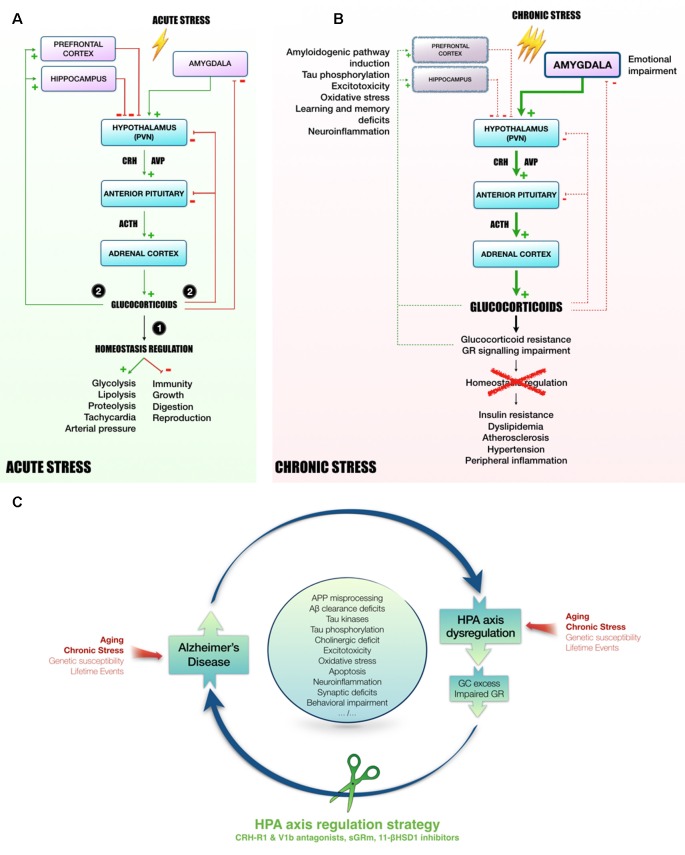
Figure 1.
Mechanisms linking HPA axis dysregulation and AD. Following acute stress (A), hypothalamic PVN releases CRH and AVP in the blood portal of median eminence. In response to CRH and AVP, corticotropic cells of anterior pituitary release ACTH in the peripheral circulation to induce GC secretion in blood by adrenal cortex. Succinctly, (1) GC mobilize energy resources and increase cardiovascular function to fight stress. Besides, GC inhibit unnecessary functions in the early phase of stress response, such as immunity, growth, digestion and reproduction. Then, (2) to avoid runaway of the system, GC exert an inhibitory feedback at all stages of HPA axis (hypothalamus and pituitary). In addition, as they easily penetrate in the brain, GC also act on several regions involved in the control of HPA axis activity, such as hippocampus and prefrontal cortex (tonic inhibition) or amygdala (tonic stimulation, Canet et al., 2018). However, chronic stress leads to a sustained activation of HPA axis and could induce stress-related disorders, as for instance MMD and AD (B, Canet et al., 2018). In this context, GC over-secretion is associated with GC resistance and GR signaling impairment (Chrousos et al., 1993). Homeostasis maintenance is compromised, leading to insulin resistance, dyslipidemia, atherosclerosis, hypertension and a massive peripheral inflammation (Vitellius et al., 2018; Maslov et al., 2019). In limbic structures (hippocampus, prefrontal cortex), it was shown that GC overexposure induces hippocampal and cortical atrophy (McEwen, 2008) and amygdala hypertrophy (Vyas et al., 2003, 2004), that could be related to learning and memory deficits, emotional impairment, excitotoxicity, neuroinflammation and oxidative stress (Sapolsky, 1996; McEwen, 2008; Bengoetxea et al., 2016). In the AD context, high levels of GC, and the dysregulation of the HPA axis activity observed in patients (Hartmann et al., 1997; Swanwick et al., 1998), seems to be particularly involved in the induction of amyloidogenic pathway and the abnormal phosphorylation of Tau (Green et al., 2006; Pineau et al., 2016; Sotiropoulos and Sousa, 2016; Vyas et al., 2016; Canet et al., 2019). Thus, it appears that the rise of circulating GC increases AD pathology, resulting in a vicious cycle by which pathology induces HPA axis dysregulation, GC overexposure and GR signaling impairment, which in turn potentiates the pathology. Due to its primordial role in the maintenance of homeostasis, targeting HPA axis offers multiple angles of action to break this vicious cycle and pave the way to new therapeutic strategies (C). Abbreviations: 11-βHSD1, 11β-hydroxysteroid dehydrogenase-1; Aβ, amyloid-β protein; APP, amyloid precursor protein; ACTH, adrenocorticotropin; AD, Alzheimer’s disease; AVP, arginine-vasopressin; CRH, corticotropin releasing hormone; CRH-R1, CRH receptor type 1; GC, glucocorticoids; GR, glucocorticoid receptors; HPA axis, Hypothalamic-pituitary adrenal axis; MDD, Major depressive disorder; PVN, paraventricular nucleus; sGRm, Selective GR modulator; V1b, Arginine-vasopressin receptor sub-type 1b.