Abstract
Background:
Intravenous immunoglobulin (IVIG) is a pooled human plasma protein that has shown efficacy in treating a variety of disorders. IVIG is generally well tolerated and has a good safety profile. There are various IVIG products available on the market, which results in differences in efficacy and safety profile. The aim of this study was to assess the safety profile of IVIG use in pediatric patients and its association with other predicted factors.
Methods:
Retrospective chart review study of all pediatric patients who received IVIG as an inpatient at Hamad General Hospital in Qatar during 2014. The occurrence of adverse drug reactions (ADR) was tested for any association with other predicted factors, such as patient age, IVIG dose, brand, and adherence to infusion protocol.
Results:
A total of 345 IVIG prescriptions were received by pediatric patients during the study period. Most common documented side effects were: fever (5.8%), chills (2.6%), and headache (2%). Renal insufficiency was observed only in six cases, with five of those in ‘Risk’ category according to RIFLE criteria. A hypersensitivity reaction was documented in seven patients, despite being premedicated with paracetamol and/or diphenhydramine and following the infusion protocol. None of the predicted factors were found to be significantly associated with ADR incidence except IVIG brand.
Conclusions:
IVIG generally has a good safety profile in pediatric patients, with low risk of severe ADR. More studies are needed to evaluate the correlation between ADR and IVIG formulation, taking into account other factors that may affect results.
Keywords: Adverse drug reaction, Immunoglobulin, Pediatrics
Introduction
Intravenous immunoglobulin (IVIG) is a pooled human plasma protein that has shown efficacy in treating a variety of disorders.1,2 The demand for IVIG is increasing steadily worldwide, and its used has evolved dramatically in the last few years, especially in pediatric patients.3
IVIG is generally well tolerated and has a good safety profile; however, its use carries the potential risks associated with all blood products.4,5 Adverse drug reactions (ADR) of IVIG infusion range from 3% to 15%, and are usually mild, self-limiting, and can be avoided by decreasing the rate of infusion.6 Thus, IVIG should be administered according to the administration protocol to avoid infusion-related side effects. Examples of IVIG adverse effects include abdominal pain, nausea, chills, rhinitis, low-grade fever, myalgia, and headaches.1,6 More serious adverse events can occur during or soon after infusion: anaphylaxis/ hypersensitivity reactions, cardiovascular events, hemolysis, and renal toxicity.6,7 Although infectious complications have not commonly occurred, cases of hepatitis C infection associated with IVIG have been reported.8,9
In 2014, over 25 IVIG preparations/brands were available worldwide and approved by various regulatory bodies.7 The various IVIG products differ in a number of ways, including IgG subclass distribution, antibody content, approved maximum infusion rate and sodium/sugar content. The characteristics of the various products may result in differences in efficacy and safety, which may have a significant impact on the choice of product for some patients.7,10 Two studies reporting the safety profile of IVIG found some differences in the incidence of ADR among different brands used.11,12 Unfortunately, the brands used in these studies were different from those available here in Qatar (except for Octagam®). In addition, there are no previous reports of ADR incidence with IVIG in pediatric patients in Qatar. Accordingly, and because safety data for use of IVIG in pediatric patients is scarce, this study was conducted.
The primary objective of this study was to assess the incidence of ADR associated with IVIG administration in pediatric patients. The secondary objectives of the study were to: determine the incidence of acute renal insufficiency associated with IVIG in pediatric patients and its severity/stages, examine the association between ADR and IVIG brand used, and investigate association between ADR and adherence to infusion protocol or other patient variable.
Methods
Study design and patients
This was a retrospective chart review study assessing the safety profile of IVIG in pediatric patients in Qatar. The pharmacy computer system was used to identify and generate a list of patients who received IVIG during 2014. All pediatric patients who received IVIG at Hamad General Hospital (HGH) during 2014 were initially included. Hamad General Hospital was chosen as it was the main tertiary hospital in Qatar providing IVIG for pediatric patients at that time. The patient list generated was screened to remove any duplicates. Patients had to fulfill both of the following criteria to be included in this study: pediatric patients (<14 years as per HGH policy definition), received IVIG in the period between 1 January 2014 and 31 December 2014. However, patients with unavailability or failure to access medical records for any reason were excluded. As some patients received IVIG multiple times during that year, safety profile was evaluated for each time separately. Thus, our research unit was set as ‘prescription’ rather than ‘patient’.
Procedure
A well-structured data collection sheet considering the research study design and objectives was designed to collect all required data. Medical records solely of identified patients were examined. We reviewed both physicians’ and nurses’ notes, medication prescriptions, medication administration charts, and any other documents in the patient’s file to complete the required data. Required information included: patient demographics (age, sex, and weight), indication for IVIG administration, dosage regimen, IVIG brand used, administration of premedication, adherence to infusion protocol, and occurrence of any adverse reactions. Adherence to infusion protocol was determined mainly based on the nurse’s documentation. If the nurse documented the correct infusion rate, or mentioned any statement indicate following the infusion protocol (i.e. ‘IVIG was infused based on the infusion protocol’, ‘infusion protocol was followed’) it was considered as adherence to infusion protocol. ADR was defined and counted as follows:
Fever: temperature >38.5 within 24 h of IVIG administration;
Thromboembolic disease (TED): documented deep vein thrombosis (DVT)/pulmonary embolism (PE) within 28 days of IVIG administration4;
Renal insufficiency: a decrease in the renal function occurred within 10 days of IVIG administration,13 classified according to RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria.14 Potential confounders of renal failure were recorded, including the use of nephrotoxic medications, the presence of hypotension, and the use of intravenous contrast dye for imaging;
Hypotension: reduction in the blood pressure less than normal range for age during IVIG administration;
Anaphylaxis: sever hypersensitivity reaction occurred within IVIG administration that required cessation of IVIG infusion and administration of medication to save life.
Other ADR were assessed mainly clinically/subjectively, and there was no means other than documentation to confirm/detect such ADR. These ADR (nausea, vomiting, headache, chills, and myalgia) were counted only if documented as occurring within 24 h of IVIG administration. Occurrence of ADR (present or not) was then tested for any association with other factors, including patient age, gender, diagnosis, IVIG dose, IVIG brand, premedication use, and adherence to infusion protocol.
Ethical considerations
This study obtained approval from the Hamad Medical Corporation–Medical Research Center (HMC-MRC) in Qatar. Data quality control (review of completeness, data verification and accuracy, security, and confidentiality of data) was performed and maintained by lead research investigators. All patient information was kept confidential in a password-protected computer, and could be accessed only by study research investigators.
Statistics
Categorical and continuous values are expressed as frequency (percentage) and mean ± SD as appropriate. Data analysis and presentation are primarily descriptive. Associations between two or more categorical variables were assessed using Chi-square test and Fisher exact test as appropriate. All p values presented are two-tailed, and p values <0.05 are considered statistically significant. All statistical analyses were done using statistical packages SPSS 22.0 (SPSS, Inc., Chicago, IL, USA).
Results
IVIG prescriptions
A total of 1065 IVIG prescriptions were initially identified using the pharmacy computer system. After removing duplications and adult prescriptions, IVIG prescriptions for pediatric patients numbered 348. Reviewing the patients’ related records excluded another three IVIG prescriptions as IVIG was discontinued before being received by these patients. The final number of IVIG prescriptions included was 345, which were prescribed and received by 120 pediatric patients during the study period (i.e. 2014). Among patients, IVIG was utilized equally between male and female patients (51.9% and 48.1%, respectively). Patient age ranged from 20 days to 14 years (median 7 years). IVIG infusion protocol was followed in 84.1%. Around half of the IVIG prescriptions (52.8%) were not preceded by administration of premedication. However, when premedication was prescribed, diphenhydramine was the most common medication used. Five brands were available and used during the study period. Privigen® and Intratect® were the most common IVIG brands used, and accounted for 79.8% of IVIG prescriptions. Table 1 summarizes the data of included prescriptions.
Table 1.
Summary of data results of included prescriptions (n = 345).
| Patients characteristics | Mean ± SD |
|---|---|
| Patient age (years) | 6.5 ± 4.5 |
| Dose (gm/kg/dose) | 0.8 ± 0.5 |
| Gender [n (%)] | |
| Male | 179 (51.9) |
| Female | 166 (48.1) |
| Indication | n (%) |
| FDA-approved | 268 (77.7) |
| Primary immunodeficiency disease | 206 (59.7) |
| ITP | 32 (9.3) |
| Kawasaki disease | 30 (8.7) |
| Non FDA-approved | 77 (22.3) |
| Opsoclonus myoclonus | 13 (3.8) |
| Dermatomyositis | 13 (3.8) |
| Sepsis/Septic shock | 10 (2.9) |
| ADME | 7 (2) |
| Chylothorax | 7 (2) |
| Myocarditis | 5 (1.4) |
| GBS | 5 (1.4) |
| Interstitial lung disease | 4 (1.2) |
| Encephalitis/Vasculitis | 3 (0.9) |
| Sever eczema | 2 (0.6) |
| HLH | 1 (0.3) |
| Renal transplant (antibody and cellular rejection) | 1 (0.3) |
| No clear indication* | 6 (1.7) |
| Adherence to the infusion protocol | n (%) |
| Adherence | 282 (81.7) |
| Nonadherence | 26 (7.6) |
| Not documented | 37 (10.7) |
| Premedication used $ | n (%) |
| Paracetamol | 116 (33.6) |
| Diphenhydramine | 139 (40.3) |
| Hydrocortisone | 6 (1.7) |
| None | 182 (52.8) |
| IVIG Brand | n (%) |
| Privigen® | 182 (52.8) |
| Intratect® | 93 (27) |
| Octagam® | 58 (16.8) |
| Gammaplex® | 6 (1.7) |
| Pentaglobin® | 6 (1.7) |
There were six IVIG prescriptions ordered for unknown or with no clear indication documentation.
Some patients received more than one premedication at the same time, so sum will not add up to 100%.
ADME, Acute disseminated encephalomyelitis; FDA, United States Food and Drug Administration; GBS, Guillain–Barré syndrome; HLH, hemophagocytic lymphohistiocytosis; ITP, idiopathic/immune-mediated thrombocytopenic purpura; IVIG, intravenous immunoglobulin.
Adverse effects
IVIG was found to have a good safety profile, with IVIG infusions completed with no reaction or documented ADR in 88.1% of cases (304/345). Some patients had more than one documented ADR associated with the same IVIG infusion. The most commonly documented ADR was fever (5.8%), followed by chills (2.6%), vomiting (2.6%), and headache (2%). A hypersensitivity reaction was documented in seven patients, despite the fact that they were premedicated with paracetamol and diphenhydramine and received IVIG according to the infusion protocol. Other adverse reactions are reported in Table 2.
Table 2.
ADR associated with IVIG (n = 345).
| ADR | Headache | Fever | Hypotension | Anaphylaxis/hypersensitivity | Myalgia | Chills | Nausea/vomiting | Renal injury | TED | No reaction |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 7 | 20 | 2 | 7 | 0 | 9 | 9 | 6 | 0 | 304 |
| % | 2 | 5.8 | 0.6 | 2 | 0 | 2.6 | 2.6 | 1.7 | 0 | 88.1 |
ADR, adverse drug reactions; IVIG, intravenous immunoglobulin; TED, Thromboembolic disease.
The incidence of renal insufficiency associated with IVIG administration seemed to be low, as only six cases (1.7%) developed renal insufficiency. Five out of the six patients who developed renal insufficiency were in the ‘Risk’ category according to RIFLE criteria. All patients who developed renal insufficiency had at least two confounding factors (mainly use of other nephrotoxic medications) that may also have affected their kidney function. The IVIG doses for patients who developed renal insufficiency were within normal range, ranging from 0.34 to 2 gm/kg.
Correlation of ADR with other factors
IVIG brand
Hypersensitivity occurred only in patients received IVIG of the brands Intratect® and Privigen®. There was a statistically significant difference in overall ADR incidence between different IVIG brands used (p < 0.0001). Generally, it was noticed that Octagam® and Gammaplex® were associated with fewest ADR compared with other brands (Table 3). However, we should keep in mind that, beside the formulation/brand being used, the patient’s clinical condition, diagnosis, concurrent medications, and other factors can greatly affect the incidence of ADR.
Table 3.
ADR incidence in different IVIG brands.
| Brand | n | ADR | Hypersensitivity | No ADR | |||
|---|---|---|---|---|---|---|---|
| Pentaglobin® | 6 | 3 | 50% | 0 | 0% | 3 | 50% |
| Intratect® | 93 | 24 | 25.8% | 5 | 5.4% | 69 | 74.2% |
| Privigen® | 182 | 14 | 7.7% | 2 | 1.1% | 168 | 92.3% |
| Octagam® | 58 | 0 | 0% | 0 | 0% | 58 | 100% |
| Gammaplex® | 6 | 0 | 0% | 0 | 0% | 6 | 100% |
ADR, adverse drug reactions; IVIG, intravenous immunoglobulin.
Adherence to infusion protocol
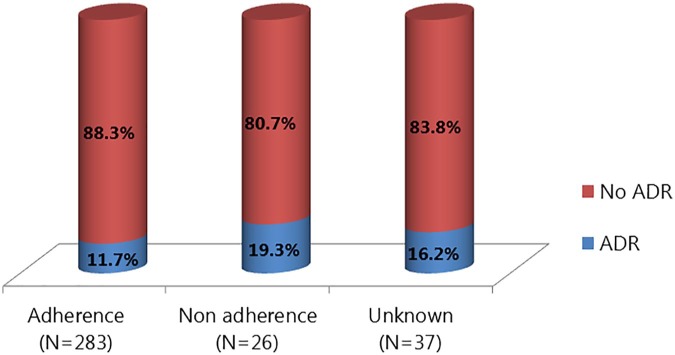
Higher ADR incidence was associated with nonadherence to IVIG infusion protocol (infusion rate) compared with adherence to infusion protocol, although this was not statistically significant (19.3% versus 11.7%, p = 0.264). No documentation of infusion protocol used was found in the patients’ medical records for 37 IVIG prescriptions (Figure 1).
Figure 1.
Incidence of ADR related to the infusion protocol adherence.
ADR, adverse drug reaction.
Patient variability
Other patient information was tested for association with ADR, including age, gender, diagnosis, IVIG dose, and premedication use. None of the these factors was found to be significantly associated with ADR incidence. However, ADR seems to be linked to high IVIG dose, as patients who developed ADR received IVIG with mean dose of (1.3 gm/kg) while the mean dose of patients without ADR was (0.78 gm/kg).
Discussion
The aim of this study was to describe the safety profile of IVIG in pediatric patients at HGH during 2014. There are very few published reports on the in depth safety profile of IVIG in pediatric populations. Our findings showed that IVIG is well tolerated and has a good safety profile in pediatric patients, with an ADR incidence of 11.9% among 345 IVIG infusions. This result is very similar to the ADR incidence (11%) reported in multicenter surveillance of IVIG use in US academic health centers.15 Additionally, our findings are also consistent with the range of systemic ADR, which has been reported to range from 3% to 15%.7 On the other hand, this incidence is much lower than that found in a study in an academic medical care in Iran that reported the occurrence of ADRs in 45.8% of infusions, which was attributed by the authors to the nurses’ negligence of the infusions protocols, brands used, and population sensitivity.16 Furthermore, one study in a Pediatric Intensive Care Unit (PICU) described ADRs of 38.8%, a higher prevalence than our findings that could be explained by the nature of PICU patients and their comorbidities.1 Similarly, recent comparable studies have shown higher ADR incidence (25.2% and 48%) due to IVIG infusions in pediatric patients.11,12 This difference could be due to the big difference in patients’ characteristics, IVIG indications, and IVIG brands used. For example, in our study, around 60% of IVIG infusions were used for primary immune deficiency (PID), while in the cited studies, only 7% received IVIG for PID indication.
In terms of the severity of the ADRs, mild and nonanaphylactoid reaction (typically fever, chills, vomiting, and headache) were more prominent in this study than other severe ADR (hypersensitivity and renal insufficiency), which is compatible with data from a previous review and another, similar, study.7,16 Renal injury associated with IVIG administration was reported in only one study of adult intensive care unit (ICU) patients, where it occurred in 81% of patients, with 21% requiring renal replacement therapy.4 Only 10% of the patients who developed renal injury had no obvious concurrent renal insult other than IVIG.4 In our study, only 1.6% had renal injury, of whom all in an ICU and had other factors affecting kidney function.
Different factors are believed to be associated with development of ADR.6 In our study, although not statistically significant, adherence to infusion protocol seems to decrease the frequency of ADRs in comparison with nonadherence. This was also confirmed in one study where three out of six patients who developed ADRs was able to complete the course of IVIG infusions after changes in IVIG infusion regimens.16 As the rate of infusion can influence the occurrence of adverse reactions, slow infusion rates with gradual stepwise increases are suggested by all IVIG drug manufacturers.7,10 In our institution, there is an administration protocol that should be followed for all patients receiving IVIG.
Because different IVIG preparations have different properties (e.g. product osmolality, sugar content, and IgA content), choice of IVIG preparation should be considered carefully as it could influence the incidence of ADR.17 Among the various IVIG preparations available locally, Privigen® was the most frequently used brand, and showed a good safety profile after Octagam® and Gammaplex®. Studies reporting the incidence of ADR associated with IVIG infusion in pediatrics used IVIG brands different from those available in Qatar11,12 except for Octagam®. In our study, we found no ADR associated with Octagam® infusion, while in another study, incidence of ADR with Octagam® was 9.5% (11/115).12 Of note, these results should be considered in the light of the difference in ability to tolerate IVIG infusion without experiencing adverse effects between one patient and another, patient clinical condition, concurrent medications, and other factors that could also affect ADR incidence beside the brand being used.17 Unfortunately, no previous study has previously tested or reported a correlation between IVIG brand used and incidence of ADR. Only one study that did mentioned the IVIG brands used stated that there was no statistical difference between the two available brands regarding adverse events causation; however, it did not mention which brands had been used and tested.1 In fact, the nature of IVIG, as a biological product derived from blood products, would lead to the expectation of some adverse reactions despite appropriate precautions and administration techniques.6,17
Limitations of our study were mainly due to its retrospective file review design, with all associated disadvantages of this study type (e.g. missing information, poor documentation, etc). However, this study is the first to report the incidence of renal failure associated with IVIG in a pediatric population, and to test for correlation between different factors and the incidence of ADR.
Conclusion
IVIG therapy is well tolerated in pediatric patients, with a good safety profile apart from mild ADRs that can occur with its administration. Renal insufficiency is rare and occurred only when patienst had other risk factors. Although none of the tested variables was found to be associated with ADR except IVIG brand, a further large-scale prospective study is required to confirm this association, taking into account other factors that may affect results.
Footnotes
Authors Contributions: R.E. contributed to design, generate patients list, and analysis and interpretation of data. R.E, A.E, and H.A contributed to acquisition of data. R.E and A.E wrote the article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: This study was funded by Hamad research center allied to Hamad Medical Corporation. This study was not funded by any pharmaceutical industry.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Reem Elajez  https://orcid.org/0000-0001-7824-3203
https://orcid.org/0000-0001-7824-3203
Contributor Information
Reem Elajez, Hamad Medical Corp, P.O Box 3050, Doha, Qatar.
Asmaa Ezzeldin, Hamad Medical Corp, Doha, Qatar.
Hossamaldein Gaber, Hamad Medical Corp, Doha, Qatar.
References
- 1. Galal NM. Pattern of intravenous immunoglobulins (IVIG) use in a pediatric intensive care facility in a resource limited setting. Afr Health Sci 2013; 13: 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darabi K, Abdel-Wahab O, Dzik WH. Current usage of intravenous immune globulin and the rationale behind it: the Massachusetts General Hospital data and a review of the literature. Transfusion 2006; 46: 741–753. [DOI] [PubMed] [Google Scholar]
- 3. Alangari AA, Abutaleb MH, Albarraq AA, et al. Intravenous immunoglobulin utilization in a tertiary care teaching hospital in Saudi Arabia. Saudi Med J 2008; 29: 975–979. [PubMed] [Google Scholar]
- 4. Foster R, Suri A, Filate W, et al. Use of intravenous immune globulin in the ICU: a retrospective review of prescribing practices and patient outcomes. Transfus Med 2010; 20: 403–408. [DOI] [PubMed] [Google Scholar]
- 5. Jolles S, Sewell WAC, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol 2005; 142: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arthur JS, Melvin B. Intravenous immune globulin: adverse effects, https://www.uptodate.com/contents/intravenous-immune-globulin-adverse-effects (accessed 8 April 2015).
- 7. Prasad AN, Chaudhary S. Intravenous immunoglobulin in pediatrics: a review. Med J Armed Forces India 2014; 70: 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Constantine MM, Thomas W, Whitman L, et al. Intravenous immunoglobulin utilization in the Canadian Atlantic provinces: a report of the Atlantic Collaborative Intravenous Immune Globulin Utilization Working Group. Transfusion 2007; 47: 2072–2080. [DOI] [PubMed] [Google Scholar]
- 9. Dodd RY. Infectious risk of plasma donations: relationship to safety of intravenous immune globulins. Clin Exp Immunol 1996; 104(Suppl. 1): 31–34. [PubMed] [Google Scholar]
- 10. Arthur JS, Melvin B. General principles in the use of immune globulin, http://www.uptodate.com/contents/general-principles-in-the-use-of-immuneglobulin? (accessed 3 April 2015). [Google Scholar]
- 11. Frances R, Shirley L, Florecita R. Adverse events of intravenous immunoglobulin infusions: a ten-year retrospective study. Asia Pac Allergy 2013; 3: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaba S, Keskindemirci G, Aydogmus C, et al. Immediate adverse reactions to intravenous immunoglobulin in children: a single center experience. Eur Ann Allergy Clin Immunol 2017; 49: 11–14. [PubMed] [Google Scholar]
- 13. Cantu TG, Hoehn-Saric EW, Burgess KM, et al. Acute renal failure associated with immunoglobulin therapy. Am J Kidney Dis 1995; 25: 228–234. [DOI] [PubMed] [Google Scholar]
- 14. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 2004; 8: R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Connie C, Lynne H, Thomas A, et al. A multicenter drug use surveillance of intravenous immunoglobulin utilization in US academic health centers. Ann Pharmacother 2000; 34: 295–299. [DOI] [PubMed] [Google Scholar]
- 16. Dashti-Khavidaki S, Khalili H, Farshadi F, et al. , Inpatient paediatric use of intravenous immunoglobulin at an academic medical centre. Singapore Med J 2008; 49: 147–149. [PubMed] [Google Scholar]
- 17. Palabrica FR, Kwong SL, Padua FR. Adverse events of intravenous immunoglobulin infusions: a ten-year retrospective study. Asia Pac Allergy 2013; 3: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]