Abstract
Background
Substantial research is dedicated to understanding the aging-related dynamics among individual differences in level, change, and variation across physical and cognitive abilities. Evaluating replicability and synthesizing these findings has been limited by differences in measurements and samples, and by study design and statistical analyses confounding between-person differences with within-person changes. In this article, we conducted a coordinated analysis and summary meta-analysis of new results on the aging-related dynamics linking pulmonary function and cognitive performance.
Methods
We performed coordinated analysis of bivariate growth models in data from 20,586 participants across eight longitudinal studies to examine individual differences in baseline level, rate of change, and occasion-specific variability in pulmonary and cognitive functioning. Results were summarized using meta-analysis.
Results
We found consistent but weak baseline and longitudinal associations in levels of pulmonary and cognitive functioning, but no associations in occasion-specific variability.
Conclusions
Results provide limited evidence for a consistent link between simultaneous changes in pulmonary and cognitive function in a normal aging population. Further research is required to understand patterns of onset of decline and differences in rates of change within and across physical and cognitive functioning domains, both within-individuals and across countries and birth cohorts. Coordinated analysis provides an efficient and rigorous approach for replicating and comparing results across independent longitudinal studies.
Keywords: Pulmonary, Cognition, Cognitive aging, Normative aging, Longitudinal analysis
Examining the dynamics of health and aging in longitudinal data is critical for understanding normal and abnormal or disease-related processes, as well as the influence of shared risk factors (1,2). Pulmonary function and cognition are two commonly used longitudinal biomarkers of aging (3). A majority of cross-sectional studies relate lower pulmonary function with lower cognitive performance in individuals across the adult lifespan, and results have suggested a more pronounced association amongst older individuals; however, few studies have thoroughly investigated the longitudinal relation between pulmonary and cognitive functioning and those that have reported inconsistent findings (4).
Numerous challenges must be considered in evaluating the dynamic relation between cognition and pulmonary function in individuals over time (2). While it is clear that both cognitive and physiological functioning, including pulmonary function, decline on average with age, associations based on cross-sectional designs and analysis can arise due to age-related mean differences alone (5). Non-aging-related factors, such as differences in environment and childhood development, contribute to early life (ie, baseline) cognitive and pulmonary differences. Longitudinally, rates of change in pulmonary and cognitive measures are known to vary due to physiological aging differences as well as to broader factors including age, education, race/ethnicity, occupational attainment, activity level, anthropometric measures, and presence of genetic or clinical pathology (4). Considering these complexities, only longitudinal data and rigorous methodological designs including key covariates can be used to disentangle if and how associations among rates of change arise from associated decline (eg, decrease in pulmonary functioning and cognitive decline occur together), common causal processes (eg, pollutant exposures damaging lungs and inciting neuroinflammation), and/or a consistent aging process (eg, normal aging resulting in aging across multiple domains of functioning/systems rather than pathological decline in targeted domains/systems) (6).
Broadening investigation to include multiple research samples, associations between biomarkers of aging, including those between physiological or cognitive processes, can be addressed in two general approaches: a pooled-data approach or a coordinated analysis approach. Data-pooling and traditional meta-analytic approaches combine information into a single data frame, whereas coordinated analysis involves conducting parallel analyses of independent studies without necessarily sharing person-level data or requiring fully harmonized measurements. While each approach has numerous strengths and weaknesses (eg, statistical power to detect effects, ease with which moderation and mediation analyses can be done, comparison of effects across individual studies, and study replication), choosing to implement one over the other perhaps most depends on measurement heterogeneity across studies. When examining the dynamics between cognitive and pulmonary change over time, heterogeneity in measurements occurs within three domains: pulmonary function measures, cognitive measures, and essential covariates. The most commonly used pulmonary outcomes include forced expiratory volume in 1 second (FEV1; ie, the volume of air an individual can forcibly exhale during the first second after maximum inhalation, obtained with spirometry) and peak expiratory flow (PEF; the maximum rate of exhalation after maximum inhalation, obtained with a peak flow meter). These measures have been demonstrated to decline at unique rates (with different patterns of change occurring at different timepoints in the lifespan) and to change differently for men and women (7,8); however, studies rarely include multiple pulmonary outcomes (4). Further, heterogeneity in rates and patterns of decline across cognitive domains (eg, fluid abilities vs crystallized abilities) has been consistently reported, and baseline and longitudinal cognitive and pulmonary assessments have been differentially associated with many factors (eg, age, education, sex, race/ethnicity, occupational attainment, activity level, anthropometric measures, and presence of genetic or clinical pathology) but without consistent operationalization of and adjustment for these variables across studies (4). Here, in an effort to resolve the inconsistencies noted above and the paucity of longitudinal evidence, we investigate the magnitude and pattern of associations across measures of pulmonary and cognitive functions at baseline, rate of change, and occasion-specific variation over time using coordinated analysis and integrative meta-analysis across eight longitudinal studies. As all results from individual studies are new and previously unpublished, we summarize them briefly here, in addition to the overall summary meta-analysis, in order to characterize the conclusions that would be drawn from the individual studies. All results are documented in more detail in the Supplementary Documents available online.
Methods
Participants, Measures, and General Study Characteristics (Table 1 and Supplementary Tables S1 and S2)
Table 1.
Baseline Demographics and Means of the Pulmonary Measures in Each of the Studies (With Standard Deviations in Parentheses)
| Demographic | EAS | ELSA | HRS | LASA | MAP | NAS | OCTO-Twin | SATSA |
|---|---|---|---|---|---|---|---|---|
| Baseline N | 869 | 6,162 | 7,900 | 1,687 | 1,598 | 1,131 | 529 | 710 |
| Age (y) | 78.3 (5.4) | 66.01 (9.36) | 69.42 (11.36) | 68.57 (8.16) | 79.88 (7.65) | 68.30 (7.39) | 83.28 (2.65) | 65.60 (8.47) |
| Male (%) | 38.20% | 46.9% | 42.8% | 50.0% | 26.8% | 100.0% | 34.3% | 38.4% |
| Education (y) | 13.0 (3.7) | 59.2%* | 12.65 (3.09) | 9.29 (3.36) | 14.67 (3.28) | 14.26 (2.68) | 7.17 (2.35) | 34.69%* |
| Height (cm) | 163.9 (9.9) | 165.58 (9.56) | 168.22 (11.14) | 171.21 (8.5) | 162.80 (9.58) | 173.81 (6.62) | 161.87 (9.03) | 165.93 (9.78) |
| Smoking history (%) | 53.4% | 63.8% | 13.8% | 24.1% | 41.86% | 76.6% | 40.0% | 46.0% |
| Cardiovascular disease (%) | 16.8% | 11.2% | 26.2% | 24.4% | 15.11% | 26.3% | 48.6% | 13.2% |
| Diabetes (%) | 16.8% | 6.3% | 17.8% | 5.3% | 19.93% | 9.1% | 9.4% | 3.4% |
| Pulmonary | ||||||||
| PEF (L/min) Mean | 287.2 (107.6) | - | 331.23 (125.76) | 427.72 (122.03) | - | - | 324.84 (107.46) | - |
| FEV1 (L/s) | - | 2.34 (0.85) | - | - | 1.67 (0.58) | 2.59 (0.60) | - | 2.23 (0.71) |
Notes: EAS = Einstein Aging Study; ELSA = English Longitudinal Study of Aging; FEV1 = forced expiratory volume in 1 second; HRS = Health Retirement Study; LASA = Longitudinal Aging Study Amsterdam; MAP = Memory and Aging Project; NAS = Veterans Affairs Normative Aging Study; OCTO-twin = Octogenarian Twin Study; PEF = peak expiratory flow; SATSA = Swedish Adoption Twin Study of Aging.
*% with ≥Grade 12 education equivalent for ELSA and % with ≥ elementary education for SATSA.
We coordinated analyses across eight different longitudinal studies with longitudinal and concurrent measurement (≥3 waves) of cognitive and pulmonary function from the Integrative Analysis of Longitudinal Studies of Aging and Dementia network (9,10). All studies affiliated with the network at the inception of this project were invited to participate. Those with longitudinal pulmonary and cognitive data, and available to participate in the project, included the Einstein Aging Study (EAS) (11), English Longitudinal Study of Ageing (ELSA) (12), Health and Retirement Study (HRS) (13,14), Longitudinal Aging Study Amsterdam (LASA) (15), Memory and Aging Project (MAP) (16,17), Veterans Affairs Normative Aging Study (NAS) (18), OCTO-Twin Study (OCTO-Twin) (19,20), and Swedish Adoption/Twin Study of Aging (SATSA) (21,22). Descriptions of the studies are provided in Supplementary Table S1, and details on baseline demographics and pulmonary measures are presented in Table 1 and Supplementary Table S2 for all cognitive measures. This represents approximately 20% of Integrative Analysis of Longitudinal Studies of Aging and Dementia affiliated studies at the time, and 80% of those meeting the data requirements. All studies have institutional ethics approval and all participants across studies provided written consent. This coordinated analysis study was approved by the University of Victoria Human Ethics Board (Protocol 09-227).
The eight independent studies are based in four countries (England, The Netherlands, United States, and Sweden). Participants with dementia were excluded at baseline. Combined, there were 20,586 participants at baseline, with samples sizes ranging from 529 (OCTO-Twin) to 7,900 (HRS). Further details are presented in Table 1. Sample sizes and descriptive statistics vary slightly for each wave and each pulmonary and cognitive pairing due to different incomplete data patterns across studies (ie, not all participants have all measures at all waves). While rate of missing data or attrition varied somewhat across each subsample (male, female) and outcome (each cognitive or physical measure), they were typical of many longitudinal studies, with retention somewhat lower for men than for women (Supplementary Tables S2 and S3). All studies included either five or six waves of longitudinal data, except ELSA (three waves, though spaced as five due to collection of physical data at every other wave). With respect to the data waves used for this article, data were collected every 1 to 4 years over approximately 5 (EAS, HRS) to 18 (NAS, SATSA) years.
Pulmonary function was assessed in each study per standard protocols using either spirometry (forced expiratory flow in 1 second; FEV1) or PEF. Half of the studies used PEF and half used FEV1. Number of cognitive measures within a study ranged from two (ELSA) to 19 (MAP) and all cognitive measures were administered per protocol.
Based on extant literature and measure availability across studies, we identified seven covariates of potential importance which could be applied consistently across all 32 samples and specified them in as similar a way as possible to facilitate interpretation of results across studies. These time-invariant covariates were baseline age, centered at 70 years, education, centered at 7 years (to accommodate lower average years of education in the European studies), height in centimeters, centered at 172 for men and 160 for women, and health characteristics at baseline, each with reference category “no”: smoking (Ever smoked?), cardiovascular disease (Ever diagnosed with myocardial infarction, angina or heart failure?), and diabetes mellitus (Ever diagnosed?). Two studies had alternative coding of education: ELSA, originally coded nominally, was dichotomized between less than and completion of high school, and SATSA, with four ordinal categories, was centered at having completed elementary level.
Most studies included more women (50%–75%), except for NAS, which was 100% men. For the six studies reporting education in years completed, the means at baseline ranged from 7 (OCTO-Twin) to 15 years (MAP). More than half of ELSA participants had the equivalent of high school education or higher. In SATSA, 35% of participants had more than an elementary education. Participants at baseline with any history of smoking ranged from 14% (HRS) to 77% (NAS). Most studies had low rates of cardiovascular disease history at baseline (11%–26%) except OCTO-Twin (49%), which consisted entirely of individuals over 80 years of age. History of diabetes at baseline was low to moderate across studies, ranging from 3% (SATSA) to 21% (MAP).
Statistical Analysis
To examine the independent and interactive associations between changes in pulmonary function and changes in cognition over time, we fit the same set of nested bivariate multilevel linear growth models describing change as a function of time since baseline assessment in each of the eight longitudinal studies, including baseline age and gradually adding the remaining covariates. Given substantial differences between the sexes on pulmonary measures, we fit models separately for men and women. To help with interpretation of cognitive measures across studies, we grouped them into traditional neuropsychological domains using their underlying theoretical and psychometric properties: mental status, processing speed, attention and working memory, perceptual reasoning, verbal ability, and learning and memory (34). Given the large variety of cognitive measures used across these studies, this method is preferable over factor analysis for two main reasons: (a) the measures are already well-validated instruments for measurement within their established cognitive domain, and (b) this method prevents erroneous factorization. All eight studies included at least one measure of learning and memory. Six studies had at least one measure of attention and working memory or perceptual reasoning. Five studies included at least one measure of mental status, processing speed, or verbal ability. Twin status was accounted for in OCTO-Twin, but not in SATSA analyses. Previous work has shown no difference in estimates of rates of change with and without adjustment.
Multivariate growth curve models provide estimates of covariation among individual differences in initial status (ie, performance at wave one), rates of linear change, and occasion-specific variability (ie, within-person correlation). The bivariate multilevel growth model essentially summarizes each person’s repeated-measure data in terms of a regression line, with estimates of everyone’s intercept, slope, and occasion-specific residual used as the outcomes in simultaneous analyses. Within-person correlations among occasion-specific residuals provide information about state-like variation after adjustment for individual differences in level and rate of change. We compared model estimates across studies and model specifications for each of the cognitive measures. To compare the effects across studies varying in sample size and in the metric of the measures, we converted covariances into correlations.
We calculated descriptive and summary statistics using software available to researchers for each study, including R and appropriate packages (23–26), SAS (27), IBM SPSS statistics (28), and Microsoft Excel. All models were estimated using Mplus (29) and output was extracted and aggregated using R (23). Results of model estimation are stored on the dedicated GitHub repository, a public cloud location facilitating transparency and reproducibility (https://github.com/IALSA/ialsa-2017-portland/blob/master/README.md) or available by request.
Meta-analytic summary
In order to summarize the results across samples and derive an overall cross-study result, we followed Cooper (30), who suggests using meta-analytic techniques to condense results from multiple studies into digestible findings in a validated way that is both robust and clear about the level of and possible sources for error. Meta-analysis uses estimates provided from each model to estimate a variance-weighted mean effect (31). Because comparison across studies relies on correlation coefficients, which are not normally distributed and are truncated at both −1 and 1, we were unable to rely on existing meta-analytic packages to create forest plots. Since prior work has shown meta-analyses relying on Fisher’s Z-transformed correlations are less biased than those reporting means derived from correlation coefficients (32), we calculated meta-analytic means using estimated Fisher’s Z statistic and then back-transformed results to correlation coefficients for the purposes of reporting. We stratified all results by sex and provided stratified means both by domain and sex. Random-effects meta-analysis was utilized in all analyses (33). I2 was calculated to examine levels of heterogeneity of effects, where appropriate.
We structured the models to be as similar as possible across studies, which is an advantage since heterogeneity in model specification can be large in the published literature and has often been cited as a reason to not conduct a meta-analysis. However, we relied on a variety of outcome measures that are similar only at the construct level, which could reasonably be expected to increase heterogeneity across studies. Furthermore, in order to gauge consistency within samples, we included multiple measures within particular domains for any study where they were available.
Results
Meta-analytic Summary Results
The estimated mean correlations from the eight studies for men and women are reported by domain in Figures 1–6 (slopes) and for all domains (slopes) in Figure 7, as well as in Supplementary Figures S1 (intercepts) and S2 (residuals). Overall, these results indicate small cross-sectional associations (mean correlations different from zero) between pulmonary and cognitive function at baseline (Supplementary Figure S1) for all domains (r = .11 to .21; overall r = .14).
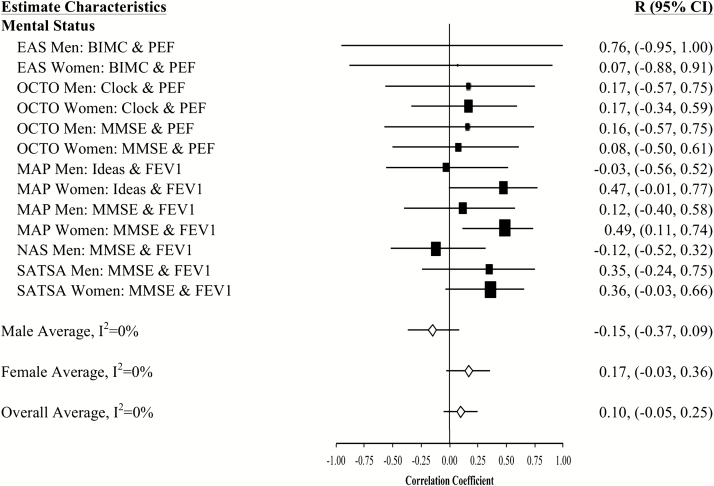
Figure 1.
Forest plot of slope (longitudinal) associations between pulmonary functioning and mental status. This figure provides estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. Sex-specific and total aggregate correlations are provided for the domain, as well as 95% bootstrap confidence intervals (CIs) for all estimates. Correlations are organized by pulmonary measure type (PEF or FEV1) and then by study (alphabetically).
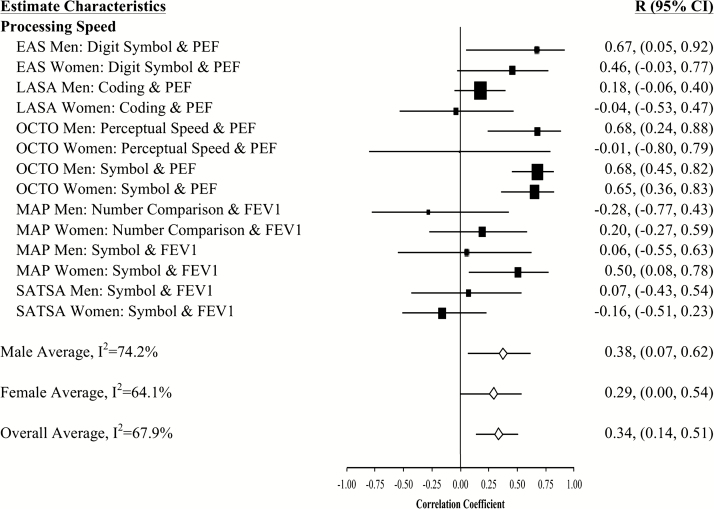
Figure 2.
Forest plot of slope (longitudinal) associations between pulmonary functioning and processing speed. This figure provides estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. Sex-specific and total aggregate correlations are provided for the domain, as well as 95% bootstrap confidence intervals (CIs) for all estimates. Correlations are organized by pulmonary measure type (PEF or FEV1) and then by study (alphabetically).
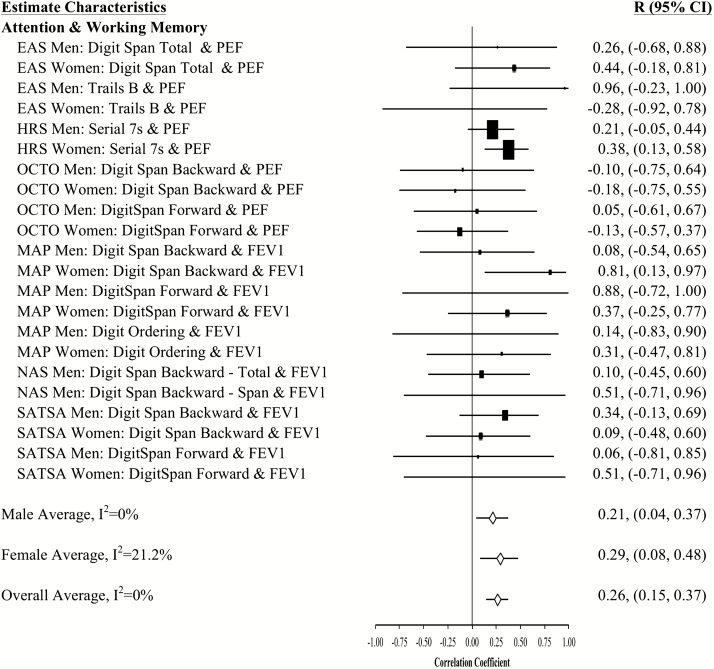
Figure 3.
Forest plot of slope (longitudinal) associations between pulmonary functioning and attention and working memory. This figure provides estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. Sex-specific and total aggregate correlations are provided for the domain, as well as 95% bootstrap confidence intervals (CIs) for all estimates. Correlations are organized by pulmonary measure type (PEF or FEV1) and then by study (alphabetically).
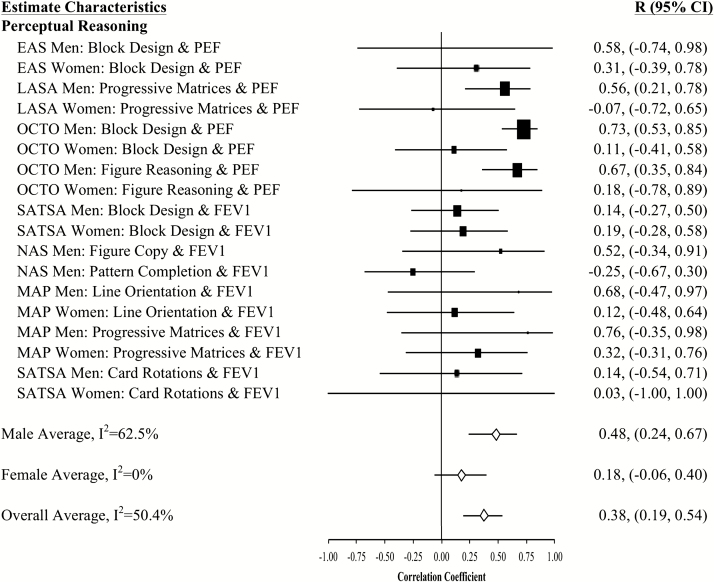
Figure 4.
Forest plot of slope (longitudinal) associations between pulmonary functioning and perceptual reasoning. This figure provides estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. Sex-specific and total aggregate correlations are provided for the domain, as well as 95% bootstrap confidence intervals (CIs) for all estimates. Correlations are organized by pulmonary measure type (PEF or FEV1) and then by study (alphabetically).
Figure 5.
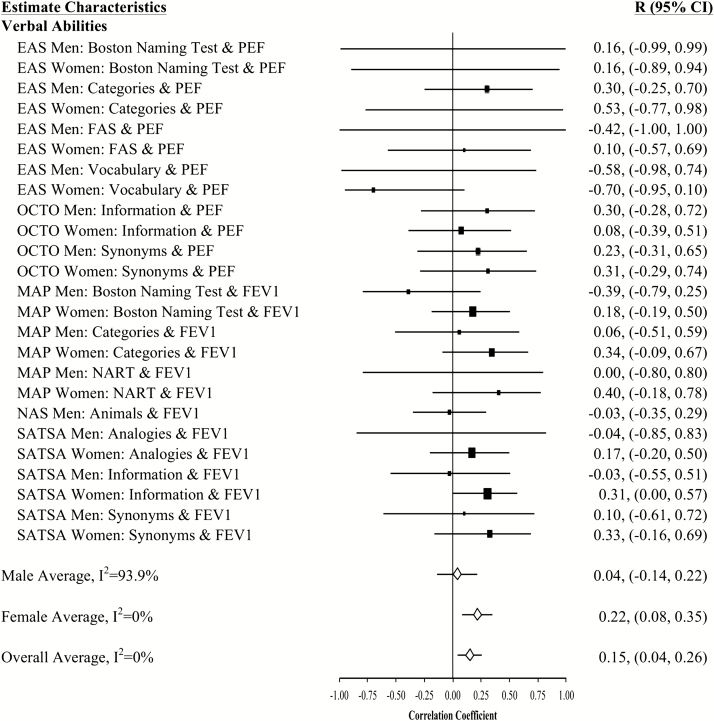
Forest plot of slope (longitudinal) associations between pulmonary functioning and verbal abilities. This figure provides estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. Sex-specific and total aggregate correlations are provided for the domain, as well as 95% bootstrap confidence intervals (CIs) for all estimates. Correlations are organized by pulmonary measure type (PEF or FEV1) and then by study (alphabetically).
Figure 6.
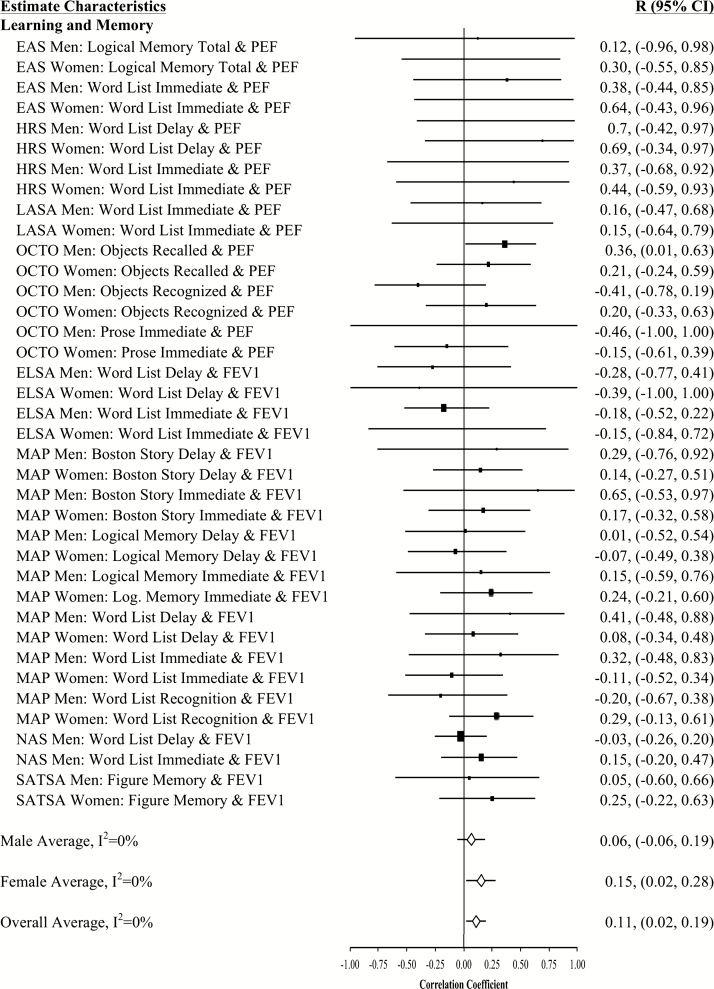
Forest plot of slope (longitudinal) associations between pulmonary functioning and learning and memory. This figure provides estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. Sex-specific and total aggregate correlations are provided for the domain, as well as 95% bootstrap confidence intervals (CIs) for all estimates. Correlations are organized by pulmonary measure type (PEF or FEV1) and then by study (alphabetically).
Figure 7.
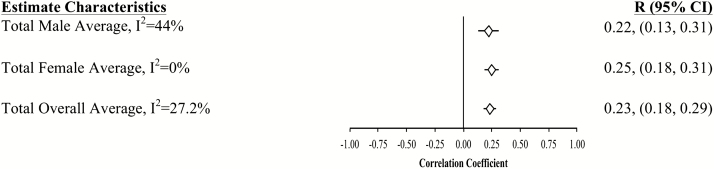
Forest plot of slope (longitudinal) associations between pulmonary functioning and all cognitive measures. This figure provides sex-specific and total aggregated estimated mean slope correlations from the eight studies (N = 20,586) for men and women are reported by domain. The 95% bootstrap confidence intervals (CIs) for all estimates are also reported.
Aggregated longitudinal slope correlations were different from zero for all domains except mental status: moderate for perceptual reasoning (r = .38), processing speed (r = .34), attention and working memory (r = .26), and smaller for verbal ability (r = .15) and learning and memory (r = .11). The aggregated total was also significant (r = .23). Longitudinal associations were found for women but not men in three domains (mental status, verbal abilities, and learning and memory), while perceptual reasoning associations were found in men but not women (Figures 1–7).
There were no meaningful occasion-specific findings for any aggregated correlations across sexes or within domains (r = −.02 to .02), with all of the overall confidence intervals containing zero (Supplementary Figure S2).
Overall, intercept correlations were fairly similar for PEF and FEV1, but PEF associations were somewhat consistently stronger (Supplementary Table S4). For example, FEV1 intercept correlations (r = .09) were approximately half the magnitude of PEF intercept correlations (r = .21). Similarly, FEV1 slope correlations (r = .15) were approximately half the magnitude of PEF slope correlations (r = .31). Much of the difference was due to processing speed, perceptual reasoning, and learning and memory. Comparison of PEF and FEV1 is complicated, however, by the fact that the four studies with PEF measurement also have the highest average ages.
Coordinated Analysis Across Individual Studies
While the meta-analytic results represent the most efficient summary of the results contained herein, this article reports previously unpublished results of the individual studies as well. Our goal here is twofold: to document the new results obtained from the individual datasets and to assess the likely conclusions that would have been drawn from individual studies. Overall, 130 new analyses were completed (one analysis equates to one pairing of a cognitive measure with a pulmonary measure in either men or women from one study). Relevant correlations from these 130 analyses are reported in Figures 1–6. Three sets of values are reported for each pair of pulmonary and cognitive measures. First, the correlation between the intercepts describes associations among baseline performance (ie, cross-sectional associations). Second, the correlation between the linear slopes indicates the association between the within-person rate of change in pulmonary function and the within-person rate of change in cognitive performance (ie, longitudinal associations). Third, the correlation between the residuals shows the coupling of within-person fluctuations (occasion-specific variability) in pulmonary function and cognitive performance at each occasion. Fluctuations refer to each person’s deviation from their predicted linear trajectory, and reflect occasion-specific sources of deviation, including random error. Coupling of such fluctuations reflects whether such occasion-specific deviations are correlated between measures of pulmonary function and cognition.
Baseline Associations Between Pulmonary Function and Cognitive Performance
At baseline, 47% (61 significant out of 130 total analyses) of the pulmonary-cognitive intercept associations were statistically significant, though most were relatively modest in size (total medial correlation = 0.13; domain-specific median correlations: processing speed = 0.21, perceptual reasoning = 0.20, mental status = 0.17, attention and working memory = 0.13, verbal ability = 0.11, learning and memory = 0.07). Across domains and studies, associations between pulmonary function and cognitive performance were common and in the expected direction. Among both men and women, most associations were significant for perceptual reasoning (15 of 18) and processing speed (12 of 14). Less than half of the baseline associations were significant for the domains of mental status (5 significant of 13 total domain analyses), attention and working memory (10 of 22), and learning and memory (11 of 38). The only inconsistency in terms of sex was for the association between pulmonary function and verbal ability. Among women, half of the baseline associations were significant (6 of 12), but among men almost none were (1 of 13). At baseline, significant associations were evenly split across the two pulmonary function measures and across sex. Significant baseline associations between pulmonary function and cognition were not consistent across studies: all were significant in LASA (perhaps due to their choice of cognitive domains assessed), while the rest varied.
Associations Between Rates of Within-Person Change in Pulmonary and Cognitive Function
Few associations between rate of change in pulmonary function and rate of change in cognitive performance were statistically significant (12 of the 130 analyses [9%]), but their magnitude was higher, ranging from 0.36 to 0.96. They occurred primarily for men as well as for processing speed and perceptual reasoning.
Exploring why so few associations between change in pulmonary function and cognitive performance were statistically significant, we noted that slope variances for pulmonary and cognitive measures were not always significantly different from zero (particularly for ELSA and LASA women, MAP men). Mean slopes for cognition were also not different from zero more frequently than expected (mainly EAS, but also OCTO and MAP), and in a small number of cases, performance improved. Pulmonary function declined over time in all but two adjusted models (OCTO-Twin men and EAS men and women) but did show decline in all the unconditional models except EAS, demonstrating that some of the decline is associated with the included covariates.
Association Between Occasion-Specific (Within-Person) Variation
Associations between the time-specific residuals, an indication of coupled within-person fluctuations (ie, time-specific change in pulmonary and cognitive performances at each occasion) were slightly more frequent than slope associations; however, only 14% (19 of the 130 analyses; 18 in the expected direction) were significant and there was no discernible pattern across cognitive domains or studies. In decreasing order of prevalence, these were for mental status (4 of 13 total domain analyses), processing speed (3 significant of 14), learning and memory (6 of 38, all of which were from the 20 word-list measures), perceptual reasoning (2 of 18), attention and working memory (2 of 22), and verbal ability (2 of 24; both SATSA Information). Occasion-specific fluctuations related to associations between FEV1 or PEF and cognitive performance, as well as across men and women, were evenly split. For the 23 significant pulmonary and cognitive performance coupling associations, OCTO-Twin had six and the rest ranged from one to four. We did not find any clear study by cognitive domain effects.
Potential Influences of Demographic, Lifestyle and Health Factors on Pulmonary and Cognitive Associations
In trying to better interpret our findings, we also examined all the covariate associations (available upon request). Overall, age was the covariate most consistently (but not universally) associated with lower performance and greater decline on pulmonary and cognitive measures. As expected, more education was associated with better cognitive performance and taller height was associated with better pulmonary function in all models, except among OCTO-Twin men. Furthermore, history of smoking was associated with lower pulmonary function in all analyses except those for OCTO-Twin men and SATSA men and women. Finally, histories of cardiovascular conditions and diabetes were inconsistently related to level of pulmonary and cognitive function at baseline, and even less frequently related to change in pulmonary and cognitive function over time.
Discussion
Although individuals generally experience physical and cognitive decline later in life, evidence regarding the nature and interplay of changes across these domains is scarce. A literature review of the aging-related dynamics between physical and cognitive function based on published research from 2000 to 2011 concluded most evidence relies primarily on cross-sectional research under the strong assumption that between-person age differences provide valid proxy estimates for within-person age-related change (2). Further, variations were found in the strength and consistency of evidence and several substantial knowledge gaps were identified, including use of inadequate analytic methods to appropriately separate between-person from within-person change.
In this article, we conducted a coordinated analysis across eight independent longitudinal studies of aging and combined these findings through meta-analysis, examining associations among individual differences in baseline level, rate of change, and occasion-specific variation in pulmonary function and cognitive performance. We employed a model that separates, as effectively as possible, the between-person differences and within-person change information and maximizes analysis of the study-specific information available. This work approximately quadruples the results available in the literature regarding associations among rates of change between pulmonary and cognitive function and does so in a way that maximizes the similarity of the models in order to facilitate research synthesis (4).
Cross-sectional associations were common, in agreement with previous literature, though of relatively small magnitude. In contrast, while statistically significant associations between rates of change were infrequent in the individual studies, the pattern of magnitude was notably larger and was overall statistically significant. Associations for occasion-specific variation were scarce and very small. This suggests that the existing literature, largely relying on cross-sectional assessment, may have, relative to within-person (longitudinal) findings, overestimated the consistency and underestimated the strength of aging-related association between these domains. In summary, both cross-sectional differences at baseline and longitudinal changes among pulmonary and cognitive functioning exhibit association, with larger associations across age-adjusted rates of change.
Combining these results with a recent systematic literature review (4), we do find some evidence to support associations between change in pulmonary function and change in cognition for aging adults in addition to the cross-sectional associations in baseline functioning. Our findings indicate that cross-sectional results based on age-heterogeneous samples are not always congruent with longitudinal associations between these domains, likely due to confounds and limitations of cross-sectional design and analysis described previously (5,6). This research was not designed to evaluate the causal mechanism of this association. Other research, however, points to the potential complexity of the associations between pulmonary and cognitive health (35–39) and future longitudinal research could investigate directional and common causal mechanisms (eg, Does cognitive deterioration lead to physical decline? Do small pulmonary changes lead to cognitive decline? Do cognitive and pulmonary decline more broadly serve as a biomarker of frailty, global health, or presence of environmental factors?).
A first limitation is the low estimated slope variances for many of the cognitive and pulmonary measures. Limited variance could be linked to measure sensitivity for assessing within-person changes over time; however, this would be surprising, given the robust literature demonstrating meaningful change in many of these common cognitive and pulmonary measures. Second, number of waves within a study is also related to slope reliability and power to detect change through the measurement noise (40); however, data were available for five or more waves for all studies except ELSA. Third, relative to measurement error, baseline measures may capture large individual differences (a lifetime of cumulative change) in contrast with slope estimates capturing change over relatively fewer years (between 5 and 15 in the data included here). Of the data included here, SATSA, LASA, and OCTO-Twin appear to contain the most longitudinal information, relative to their cross-sectional baseline age-heterogeneity. These issues could be explored further, for example, with growth mixture models, which identify subsets of individuals who experience different trajectories of change.
Although the meta-analysis yielded pulmonary and cognitive change associations for most domains and overall, it appeared many of these findings were driven by the four studies with the oldest average age—EAS, HRS MAP, and OCTO-Twin. It may be that associations between changes in pulmonary and cognitive performance occur primarily at older ages.
Of additional interest were the numerous baseline associations across domains, contrary to previous literature suggesting that fluid cognition (eg, speeded, working memory, and visuospatial reasoning-type measures) differences are more highly associated with pulmonary function differences (cf., 4). Thus, associations between pulmonary function and cognition appear more non-specific than previously suggested.
Regarding the meta-analysis, although we aimed to minimize heterogeneity by specifying models in the most similar way across studies, the different cognitive measures used across studies likely contribute to result heterogeneity. Including multiple, and sometimes similar, measures within each sample may also have reduced the ratio of cross-study to within-study variance, although this was not the motivation for including them. Combined with the fact that the smaller studies were more likely to have collected data on more cognitive measures and domains, and to have included more in-depth measures with greater reliability, this may have given the smaller studies greater influence in the aggregated estimates.
This study highlights the potential heterogeneity of results from complex longitudinal studies differing in measurements and sample characteristics, even while using identical analytical methods (41). Independently published results often vary widely in the design and statistical analyses. Against our expectation that FEV1 (regarded as a more sensitive overall measure of pulmonary functioning) would yield more significant findings than PEF, the latter (PEF) was more frequently associated with the significant baseline results, as well as the few longitudinal and occasion-specific findings. Since the different treatments of FEV1 and PEF (eg, using highest value or mean value based on best practices recommended at the time and place for each study) were equally split across studies, this did not seem to be a factor, despite some evidence that different treatments contribute to greater variability in pulmonary measures, particularly for PEF (42,43). Interestingly, some research has also shown FEV1 to be longitudinally more stable than PEF (44). In this article, we found no obvious pattern when considering the intercept, slope and residual variances distinguished by instrument and method. Since obtaining PEF is a simpler and less expensive method for assessing respiratory function, further research into the utility of longitudinal PEF and FEV1 measurements would be useful.
One interesting aspect of this research was the inclusion of two of the longitudinal studies (NAS (45) and SATSA (46,47)) in both this coordinated replication and our prior systematic review (4). Previously published findings from NAS (45) generally agree with our findings here, although their use of a different analytic method addressed a somewhat different question about the association between pulmonary function and cognition. Previously published findings from SATSA (46,47) based on a different analytic method reported some longitudinal associations between pulmonary function and cognition; however, when including our selected covariates and fitting bivariate linear growth models to individual measures by sex, using the original study data, these findings were not replicated in this coordinated analysis. Two key differences contribute to this discrepancy. First, the previous SATSA analysis made use of an “accelerated” longitudinal design based on age rather than time, which did not separate the cross-sectional and longitudinal information as thoroughly. Second, their analyses were not stratified by sex, which may have provided their analysis greater statistical power, particularly as the slope variance tended to be low in the male SATSA models. A third possible contributor was that previous research combined cognitive measures into factors before looking at longitudinal associations (4). In our coordinated analysis, we did not extract cognitive factors because they can sometimes obscure associations that would be identified with individual measures, and across studies the meanings of the factors could vary as much as individual measures do.
A further limitation of this research pertains to the clinical context of our pulmonary data. Presence of pulmonary disease (eg, COPD, emphysema, pulmonary fibrosis) was not included as a covariate in our models for three main reasons. First, not all studies assessed presence of pulmonary disease. Second, we expect presence of pulmonary disease to be highly associated with history of smoking, which was included as a covariate in our models. Third, excluding or controlling for pulmonary disease significantly reduces the variance of pulmonary function measurements as a function of the diagnostic criteria themselves. For instance, despite different etiologies, many common pulmonary diseases are diagnosed based on the presence of a range of symptomatology in the context of a spirometry score falling below an established cutoff (based on demographically corrected predicted values) and clinically significant spirometric change (ie, change likely indicative of the development of clinical pathology) is typically regarded as ≥15% per year. It could be that in healthy individuals, small changes in pulmonary and/or cognitive abilities may have little significance, but individuals with clinically significant changes may exhibit greater associations between physical health and cognitive measures, particularly since these measures were developed to detect clinically significant changes. While common in the clinical context, practices to test for clinically meaningful change are rare in cognitive aging research. Average rates of pulmonary function change in this study ranged from approximately 1%–5% which is more representative of normative than pathological change, though changes of up to 8% were observed. Isolating, excluding, or controlling for individuals who fall in the lower end of the pulmonary function distribution and/or have high proportions of pulmonary change over time is an area of follow-up research that could contribute to further understanding of the dynamics underlying the consistent, but weak longitudinal pulmonary and cognitive associations found in this study. Overall, consideration of clinically significant change will be critical to future investigations of longitudinal physical health and cognition associations.
There are many benefits to collaborative and coordinated analysis endeavors, most notably the opportunity for simultaneous evaluation of within-person data to test, replicate, and extend prior findings on patterns of change and life course determinants of aging, dementia, and health outcomes (9,41,48). There are also many challenges in synthesizing results on aging-related dynamics across multiple complex longitudinal studies. We have demonstrated how some of these challenges can be addressed through careful consideration of study methodologies and sample characteristics and implementation of the coordinated analysis approach (9,10,41) Further studies utilizing and expanding coordinated analysis will contribute to a better understanding of normative and abnormal or disease-related aging processes, with the hope of contributing to intervention and policy aimed at promoting healthy aging worldwide (48).
Funding
Research reported in this article was supported by the National Institute on Aging (P01-AG043362; Integrative Analysis of Longitudinal Studies of Aging and Dementia). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. E.D. was supported by the Vanier Canada Graduate Scholarship, Natural Sciences and Engineering Research Council of Canada. The Einstein Aging Study (EAS) is supported by the National Institutes on Aging (P01-AG03949, K01-AG054700), by the Sylvia & Leonard Marx Foundation, and the Czap Foundation. The Health and Retirement Study (HRS) is supported by the National Institute on Aging (U01-AG009740) and the Michigan Center on the Demography of Aging (P30-AG012846). The Longitudinal Aging Study Amsterdam (LASA) is supported by the Dutch Ministry of Health, Welfare and Sports and VU University and VU University Medical Center. The Rush Memory and Aging Project (MAP) at RUSH University is supported by the National Institute on Aging (R01-AG17917). Avron Spiro was supported by a Senior Research Career Scientist Award from the Clinical Science Research and Development Service, U.S. Department of Veterans Affairs. L.L. was supported by funding from the National Institute on Aging (K08-AG048221). The VA Normative Aging Study (NAS) is a research component of MAVERIC and is supported by the VA Cooperative Studies Program/Epidemiologic Research Centers. The views expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs or other support institutions. The OCTO-Twin study is supported by the National Institute on Aging (AG08861), the Swedish Research Council for Health, Working Life and Welfare (Epilife FAS Center and AGECAP 2013–2300), the Adlerbertska Foundation, Hjalmar Svensson Foundation, Knut and Alice Wallenberg Foundation, the Wenner-Gren Foundations, Wilhelm and Martina Lundgrens Foundation, and Swedish Brain Power Consortium. The Swedish Adoption/Twin Study of Aging (SATSA) is supported by the National Institute on Aging (AG04563, AG10175), the MacArthur Foundation Research Network on Successful Aging, the Swedish Research Council for Health, Working Life and Welfare (97:0147:1B), and the Swedish Research Council.
Supplementary Material
Acknowledgments
We gratefully acknowledge and thank the many researchers and participants who contributed the data and analyses of the eight different longitudinal studies of aging included in this article.
Conflict of interest statement
None declared.
References
- 1. Clouston SA, Brewster P, Kuh D, et al. . The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol Rev. 2013;35:33–50. doi: 10.1093/epirev/mxs004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spiro A III, Brady CB. Integrating health into cognitive aging research and theory: quo vadis? In: Hofer SM, Alwin DF eds. Handbook of Cognitive Aging: Interdisciplinary Perspectives. Thousand Oaks, CA: Sage Publications, Inc.; 2008:260–283. doi: 10.4135/9781412976589.n16 [DOI] [Google Scholar]
- 3. Lara J, Cooper R, Nissan J, et al. . A proposed panel of biomarkers of healthy ageing. BMC Med. 2015;13:222. doi: 10.1186/s12916-015-0470-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duggan EC, Graham RB, Piccinin AM, Clouston S, Muniz Terrera G, Hofer SM. A systematic review of pulmonary function and cognition in aging. J Ger Psy Sci Series B. 2018; online first, doi: 10.1093/geronb/gby128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hofer SM, Flaherty BP, Hoffman L. Cross-sectional analysis of time-dependent data: mean-induced association in age-heterogeneous samples and an alternative method based on sequential narrow age-cohort samples. Multivariate Behav Res. 2006;41:165–187. doi: 10.1207/s15327906mbr4102_4 [DOI] [PubMed] [Google Scholar]
- 6. Hofer SM, Sliwinski MJ. Understanding Ageing. An evaluation of research designs for assessing the interdependence of ageing-related changes. Gerontology. 2001;47:341–352. doi: 10.1207/s15327906mbr4102_4 [DOI] [PubMed] [Google Scholar]
- 7. Nunn AJ, Gregg I. New regression equations for predicting peak expiratory flow in adults. BMJ. 1989;298:1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67:264–275. doi: 10.1093/gerona/glr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hofer SM, Piccinin AM. Integrative data analysis through coordination of measurement and analysis protocol across independent longitudinal studies. Psychol Methods. 2009;14:150–164. doi: 10.1037/a0015566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piccinin AM, Hofer SM. Integrative Analysis of Longitudinal Studies on Aging: collaborative research networks, meta-analysis and optimizing future studies. In: Hofer SM, Alwin DF, eds. Handbook on Cognitive Aging: Interdisciplinary Perspectives. Thousand Oaks, CA: Sage; 2008:446–476. doi: 10.4135/9781412976589.n27 [DOI] [Google Scholar]
- 11. Katz MJ, Lipton RB, Hall CB, et al. . Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42:1640–1648. doi: 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crimmins E, Guyer H, Langa K, Ofstedal MB, Wallace R, Weir D.. HRS Documentation Report. Ann Arbor, MI: University of Michigan; 2008. [Google Scholar]
- 14. Heeringa SG, Connor JH.. Technical Description of the Health and Retirement Survey Sample Design. Ann Arbor, MI: University of Michigan; 1995. doi: 10.7826/ISR-M.06.585031.001.05.0001.1995 [DOI] [Google Scholar]
- 15. Huisman M, Poppelaars J, van der Horst M, et al. . Cohort profile: the Longitudinal Aging Study Amsterdam. Int J Epidemiol. 2011;40:868–876. doi: 10.1093/ije/dyq219 [DOI] [PubMed] [Google Scholar]
- 16. Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The rush memory and aging project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446 [DOI] [PubMed] [Google Scholar]
- 17. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview of findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spiro A III, Bossé R. The normative aging study. In: Maddox GL. (ed). The Encyclopedia of Aging. 3rd ed New York, NY: Springer; 2001: 744–746. [Google Scholar]
- 19. Johansson B, Hofer SM, Allaire JC, et al. . Change in cognitive capabilities in the oldest old: the effects of proximity to death in genetically related individuals over a 6-year period. Psychol Aging. 2004;19:145–156. doi: 10.1037/0882-7974.19.1.145 [DOI] [PubMed] [Google Scholar]
- 20. McClearn GE, Johansson B, Berg S, et al. . Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–1563. doi: 10.1126/science.276.5318.1560 [DOI] [PubMed] [Google Scholar]
- 21. Cederlöf R, Lorich U. The Swedish Twin Registry. In: Nance WE, Allen G, Parisi P (eds). Twin Reserach: Part C. Biology and Epidemiology. New York: Alan R Liss; 1978:189–195. [PubMed] [Google Scholar]
- 22. Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: the Swedish Adoption/Twin Study of Aging. Aging Neuropsychol C. 2004;11:325–345. doi: 10.1080/13825580490511152 [DOI] [Google Scholar]
- 23. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: The R Foundation for Statistical Computing; 2013. Downloaded from: http://www.R-project.org/. Accessed December 22, 2018. [Google Scholar]
- 24. Wickham H, RStudio Tidyverse; 2017. https://CRAN.R-project.org/package=tidyverse. Accessed December 22, 2018.
- 25. Hallquist MN, Wiley JF. MplusAutomation. Struct Equ Model. 2018:1–19. doi: 10.1080/10705511.2017.1402334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beasley W, Koval A. Integrative analysis of longitudinal studies of aging; 2015:25 https://cran.r-project.org/web/packages/IalsaSynthesis/index.html. Accessed December 22, 2018.
- 27. SAS Institute Inc. SAS 9.1.3 Help and Documentation. Cary, NC: SAS Institute Inc; 2002–2004. [Google Scholar]
- 28. IBM Corp. SPSS Statistics for Windows, Versions 22.0–24.0. Armonk, NY; 2013–2016. https://www-01.ibm.com/support/docview.wss?uid=swg21476197 [Google Scholar]
- 29. Muthén LK, Muthén BO.. Mplus User’s Guide. 7th ed Los Angeles, CA: Muthén & Muthén; 1998–2015. [Google Scholar]
- 30. Cooper R, Hardy R, Aihie Sayer A, et al. ; HALCyon study team Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS One. 2011;6:e27899. doi: 10.1371/journal.pone.0027899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 32. Silver NC, Dunlap WP. Averaging correlation coefficients: should Fisher’s z transformation be used? J Appl Psychol. 1987;72:146. doi: 10.1037/0021-9010.72.1.146 [DOI] [Google Scholar]
- 33. Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods. 1998;3:486. doi: 10.1037/1082-989X.3.4.486 [DOI] [Google Scholar]
- 34. Strauss E, Sherman EMS, Spreen O.. A Compendium of Neuropsychological Tests. New York: Oxford University Press, 2006. [Google Scholar]
- 35. Strömmer JM, Davis SW, Henson RN, Tyler LK, Cam-CAN, Campbell KL. Physical activity predicts population-level age-related differences in frontal white matter. J Gerontol A Med Sci. 2018. doi: 10.1093/Gerona/gly220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stigger FS, Zago Marcolino MA, Portela KM, Méa Plentaz RD. Effects of exercise on inflammatory, oxidative and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: a systematic review and meta-analysis. J Gerontol A Med Sci. 2018. doi: 10.1093/geroa/gly173 [DOI] [PubMed] [Google Scholar]
- 37. Maltais M, de Souto Barreto P, Hooper C, Payoux P, Rolland Y, Vellas B, MAPT/DSA Study Group Associations between brain Aβ-amyloid and frailty in older adults. J Gerontol A Med Sci. 2019. doi: 10.1093/Gerona/glz009 [DOI] [PubMed] [Google Scholar]
- 38. Lahousse L, Ziere G, Verlinden VJA, et al. . Risk of frailty in elderly with COPD: a population-based study. J Gerontol A Med Sci. 2016;5:689–695. doi: 10.1093/Gerona/glv154 [DOI] [PubMed] [Google Scholar]
- 39. Cheval D, Chabert C, Orsholits D, et al. . Disadvantaged early-life socioeconomic circumstances are associated with low respiratory function in older age. J Gerontol A Med Sci. 2018. doi:10.10.93/Gerona/gly177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rast P, Hofer SM. Longitudinal design considerations to optimize power to detect variances and covariances among rates of change: simulation results based on actual longitudinal studies. Psychol Methods. 2014;19:133–154. doi: 10.1037/a0034524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hofer SM, Piccinin AM. Toward an integrative science of life-span development and aging. J Gerontol B-Psychol. 2010;65:269–278. doi: 10.1093/geronb/gbq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller MR, Crapo R, Hankinson J, et al. . General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- 43. Pellegrino R, Viegi G, Brusasco V, et al. . Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi:0.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 44. Twisk JW, Staal BJ, Brinkman MN, Kemper HC, van Mechelen W. Tracking of lung function parameters and the longitudinal relationship with lifestyle. Eur Respir J. 1998;12:627–634. [DOI] [PubMed] [Google Scholar]
- 45. Weuve J, Glymour MM, Hu H, et al. . Forced expiratory volume in 1 second and cognitive aging in men. J Am Geriatr Soc. 2011;59:1283–1292. doi: 10.1111/j.1532-5415.2011.03487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emery CF, Finkel D, Pedersen NL. Pulmonary function as a cause of cognitive aging. Psychol Sci. 2012;23:1024–1032. doi: 10.1177/0956797612439422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Finkel D, Reynolds CA, Emery CF, Pedersen NL. Genetic and environmental variation in lung function drives subsequent variation in aging of fluid intelligence. Behav Genet. 2013;43:274–285. doi: 10.1007/s10519-013-9600-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gallacher J, Hofer SM. Generating large-scale longitudinal data resources for aging research. J Gerontol B Psychol Sci Soc Sci. 2011;66 (Suppl 1):i172–i179. doi: 10.1093/geronb/gbr047 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.