Abstract
Objective
The management of spinal aneurysmal bone cysts (ABCs) is complex and often requires multimodality therapy, including surgical intervention to stabilize the axial skeleton, and avoid neurologic injury or death. With en bloc resection, ABCs have a recurrence rate of 12%, which increases to >50% with subtotal resection. The use of doxycycline sclerotherapy has been reported to reduce the recurrence rate of non-spinal ABCs to 5% at >24 month follow-up. We retrospectively reviewed our institutional results for sodium tetradecyl sulfate (STS)/doxycycline sclerotherapy and surgical intervention for spinal ABCs, to assess our treatment paradigm for these tumors and inform our future approach to these lesions.
Methods
Three cervical, two thoracic and two lumbar spine ABCs were treated in seven patients with spine-exclusive disease at our institution from 2011 to the present. The most common presenting complaint was pain. Each patient was retrospectively reviewed for clinical symptomology, number of treatments, technique and clinical follow-up. Qualitative assessment of improvement was based on the most recent clinical evaluation.
Results
The cohort underwent a mean of three treatment sessions (range 2–15). All were treated with STS and/or doxycycline. Five patients underwent surgical intervention at some point, either before or following sclerotherapy. After the last sclerotherapy session, four patients reported stable or improved pain symptoms, while two reported progressive pain that required surgical intervention for that indication. One patient, who underwent both multiple rounds of sclerotherapy and surgical resection, died due to acute on chronic cervical spine collapse with cord compression and inability to control disease.
Conclusion
We report our experience in the treatment of spinal column ABCs. Stabilization or improvement in pain was seen in four patients, while the remainder had progressive disease. Our multidisciplinary approach allows patients to receive the most appropriate treatment at presentation and thereafter, for symptom amelioration or spinal stability. Important future goals are to quantitatively assess changes in symptoms over time and to incorporate a reproducible radiographic endpoint for the assessment of treatment efficacy.
Keywords: Aneurysmal bone cyst, ABC, aneurysmal bone cysts, ABCs, doxycycline, sclerotherapy, sodium tetradecyl sulfate, STS, spine
Introduction
Aneurysmal bone cysts (ABCs) are benign, expansile, locally aggressive tumors whose pathophysiology has been incompletely worked out, although most investigators now believe that they are the result of a vascular malformation within the bone. These malformations are driven by upregulation of the ubiquitin-specific protease USP6 (Tre2) gene on 17p13 when combined by translocation with a promoter pairing. The most commonly described translocation, t(16;17) (q22;p13), leads to juxtaposition of the promoter region CDH11 on 16q22.1,2
Three commonly proposed theories for the development of ABCs are as follows:
ABCs may be caused by a reaction secondary to another bony lesion; there is a high incidence of accompanying tumors in ABCs (23–32%), with giant cell tumors being the most common. Other tumors include fibrous dysplasia, osteoblastoma, chondroblastoma, chondromyxoid fibroma, non-ossifying fibroma and others.
ABCs may arise de novo, as ∼ 70% of ABCs arise without evidence of another lesion.
The goal of this work was not to conclude the debate on the origins of ABCs, but rather to offer a look at the evolution of the treatment of spinal ABCs and begin pointing the way to future directions.
Until approximately 5 years ago, treatment of ABCs focused primarily on surgical resection. Recently, alternative approaches to surgical therapy have been identified, including endovascular intervention6 and intralesional polidocanol.7
A single agent that was safe and effective for percutaneous intralesional administration for appendicular skeletal ABCs was not identified until 2013, when Shiels and Mayerson demonstrated that doxycycline sclerotherapy of ABCs could result in durable healing with a low recurrence rate.8 Treatment of axial skeleton ABCs with a sclerosant is yet to be thoroughly investigated and it has not yet been established as a component of the treatment algorithm.
The treatment of spinal ABCs is unique as these lesions do not lend themselves to a protracted treatment algorithm; the stabilizing and protective functions of the spinal column, which place the spinal cord at risk in the setting of instability or nerve compression, preclude a long waiting period for radiographic evidence of bone stabilization and healing. These important functions may serve to drive practitioners towards surgical intervention at a faster rate than non-axial skeletal lesions, in order to avoid permanent neurological injury due to spinal ABCs.
We reviewed our experience over the last 5 years of treating spinal ABCs, in an attempt to identify whether the use of sclerotherapy in the spine is safe as a surgical adjunct, and if sclerotherapy can result in sufficient bony healing to stabilize symptomology and prevent spinal cord injury or major complication.
Methods
A retrospective review was performed according to institutional guidelines. Institutional review board (IRB) approval was obtained. Computerized records were reviewed and analyzed for seven patients treated between September 2011 and October 2016. All participants, or their proxies, provided written and informed consent for release of their clinical data using an IRB-approved, Health Insurance Portability and Accountability Act-compliant protocol. Included cases involved isolated single-site ABCs of the cervical, thoracic or lumbar spine. No patients with this disease distribution required exclusion.
The data obtained from the medical records included demographic information, treatment protocols, imaging evaluations and clinical notes.
The indications for sclerosant therapy were: (a) recurrence after surgical curettage and bone grafting (n = 3), and (b) interdisciplinary review and agreement on percutaneous treatment as an alternative to surgical treatment (n = 4). The contraindications for surgery were patients with an inoperable site or prior debulking resulting in an unfavorable surgical bed. All lesions were centered within the vertebrae (bodies and posterior elements).
The patients ranged from 8 to 18 years of age with a mean of 15 years (Table 1). All patients underwent magnetic resonance imaging (MRI) examinations. The definitive diagnosis of ABCs was made by either pre-treatment surgical biopsy or day-of-treatment percutaneous biopsy. Imaging characteristics served as an adjunct for diagnosis. Histologic analysis confirmed the three key diagnostic features of ABC: fibroproliferative stroma, giant cell-like osteoclasts and vascular spaces.
Table 1.
Patient and treatment demographics.
| Number of patients | n = 7 |
|---|---|
| Age (years) | 8–18 (average 14, median 15) |
| Number of treatments | 2–15 (average 4.4, median 3) |
| Male:female | 4:3 |
| Sclerosing agents | Sodium tetradecyl sulfate, doxycycline |
| Surgical patients | 5 |
| Non-surgical patients | 2 |
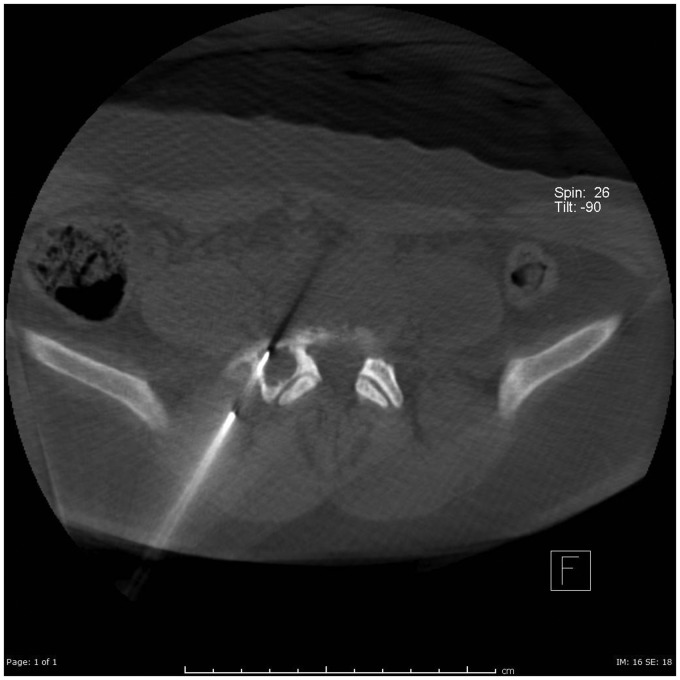
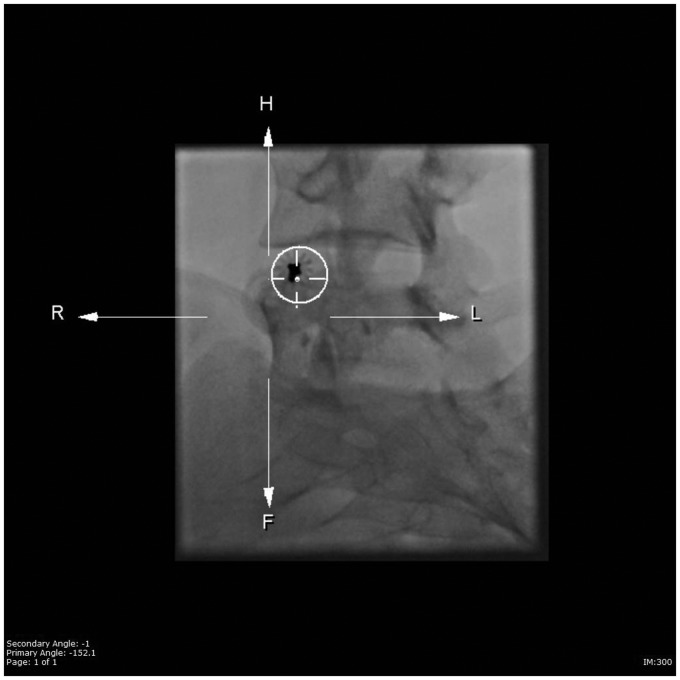
Treatment procedures were performed on an outpatient basis in the interventional radiology suite with general anesthesia. Pre-treatment consultation (and post-treatment follow-up visits) included obtaining the pertinent medical and pain history by the treating radiologist and spine surgeon. Percutaneous access, with 16- to 22-gauge needles, was performed with fluoroscopic, cone-beam computed tomography (Figure 1), and i-Guide navigation fluoroscopy guidance (Figure 2) (Siemens Medical, Munich, Germany). Needle size was based on the thickness or absence of cortical bone overlying the ABC, and on the need for a core biopsy.
Figure 1.
Dyna computed tomography image demonstrating percutaneous access into an aneurysmal bone cyst.
Figure 2.
i-Guide image demonstrating navigation fluoroscopic guidance into an aneurysmal bone cyst (anterior–Posterior end-on view allows precise trajectory of needle entry).
Contrast cystograms were performed before doxycycline injection. When contrast cystograms demonstrated rapid opacification of considerable draining veins, the entry needle was repositioned. Then, 1–2% sodium tetradecyl sulfate (STS) and doxycycline (10 mg/mL) (Fresenius Kabi, Lake Zurich, IL, USA) were used as the sclerosants and delivered as protein foam after agitation. STS alone, doxycycline alone or STS first, followed by aspiration and then doxycycline injection, were the possible treatment protocols over the length of this review (Table 2).
Table 2.
Patient treatment regimens.
| Patient number | Location | STS alone | Doxycycline alone | STS followed by doxycycline | Total treatments |
|---|---|---|---|---|---|
| 1 | T12 | 1 | 0 | 1 | 2 |
| 2 | C4 | 2 | 0 | 0 | 2 |
| 3 | L5 | 2 | 0 | 1 | 3 |
| 4 | L3 | 0 | 1 | 3 | 3 |
| 5 | C2–C4 | 0 | 12 | 3 | 15 |
| 6 | C2 | 4 | 2 | 0 | 3 |
| 7 | T8 | 2 | 0 | 0 | 2 |
STS: sodium tetradecyl sulfate.
For the STS, a mixture of STS (Mylan Institutional, Rockford, IL, USA) and Optiray 240 was agitated via two syringes and a three-way stopcock with air to create a stable foam delivery system. The STS admixture was mixed with 2–3 mL air (1:2–3 dilution ratio) and agitated between two 10-mL syringes with an interposed stopcock to make an approximately 1–2% foam concentration for injection. STS was injected into the cystic locules.
For the doxycycline, a mixture of doxycycline and 25% albumin (Grifals Biologicals, Los Angeles, CA, USA) was agitated with air to create a stable protein foam delivery system. Next, 5 mL of 25% human serum albumin was added to the doxycycline liquid in the interventional radiology suite (thus 20 mg/mL doxycycline in the albumin mixture). The doxycycline/albumin admixture was then mixed with 10 mL air and agitated between two 10-mL syringes with an interposed stopcock to ultimately inject 10 mg/mL doxycycline foam.
Multilocular spinal ABCs required 1–3 needles for each treatment. Post-procedural pain was managed with either oral non-steroidal anti-inflammatory agents or oral narcotic analgesics. Post-sclerotherapy follow-up was performed via interventional radiology and longitudinal care was coordinated by the primary surgeon. Post-treatment follow-ups ranged from 9 to 58 months with a mean of 29 months (Table 3).
Table 3.
Patient outcomes.
| Patient number | Follow-up (months) | Underwent surgery | Outcome |
|---|---|---|---|
| 1 | 9 | No | Persistent pain; braced, analgesics |
| 2 | 45 | Yes | Stable symptoms, no pain |
| 3 | 34 | No | Stable symptoms, mild pain |
| 4 | 18 | Yes | Stable symptoms, no pain |
| 5 | 12 | Yes | Stable symptoms, minimal pain |
| 6 | 26 | Yes | Death |
| 7 | 58 | Yes | Parasthesias |
Follow-up MRI studies were not regularly scheduled for all patients, although most received interval scans if there were concerns related to non-resolution/recurrence of symptoms or a change in clinical status. Treatment was considered completed when symptoms stabilized or resolved. Imaging was reviewed by at least three physicians from the treating group. Images were evaluated for qualitative evidence of: (a) fibrosis/healing of the cystic spaces and cortical thickening, or (b) evidence of recurrent bony osteolysis or an expansion during the follow-up period.
No minor or major complications related to treatment were reported within 30 days of any treatment. One patient, with a particularly aggressive lesion, expired 2 years after his last intervention due to an inability to control symptoms and stabilize the spinal column using either surgical or interventional techniques.
Results
Most patients underwent two or three treatment sessions, although the number of sessions ranged from 2–15. All were treated with STS and/or doxycycline. After the last treatment session, four patients reported stable or improved pain symptoms, while two reported progressive pain that required surgical intervention for that indication. One patient had persistent symptoms that were managed with bracing and analgesics. Five patients underwent surgical intervention at some point, either before (n = 3) or following (n = 2) sclerotherapy. Three patients reported ongoing symptoms following sclerotherapy. One patient died due to acute on chronic cervical spine collapse with cord compression and an inability to control disease progression by any treatment modality.
There were no significant complications related to any procedure.
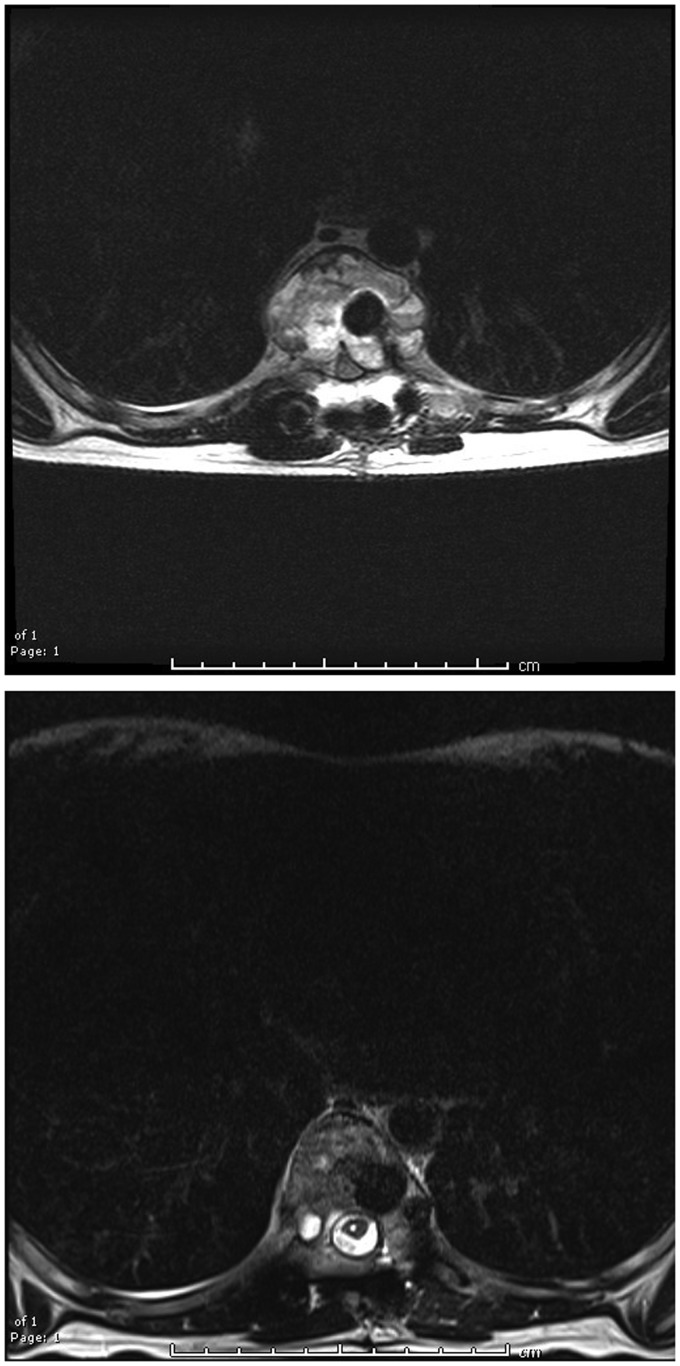
The pre- and post-treatment images from one patient demonstrate evidence of post-sclerotherapy healing of the cystic lesion (Figure 3).
Figure 3.
Pre- and post-sclerotherapy T2-weighted images demonstrating marked reduction in cyst size and re-expansion of the spinal canal; the spinal cord also decompressed.
Discussion and conclusions
ABCs are relatively rare, benign, lytic and highly vascular lesions that are generally diagnosed within the first few decades of life. They can present in any location of the axial or appendicular skeleton, and may present with a variety of symptoms, but most typically present with pain, swelling or a mass.
Histologically, ABCs are characterized by blood-filled lakes interposed between fibroproliferative stroma and contain giant cell-like osteoclasts. Historically, these have been treated surgically, with curettage and bone graft, or en bloc resection.9 However, with particular respect to ABCs of the spine, there have been efforts in recent years to try to incorporate adjunctive or primary minimally invasive treatments in an attempt to avoid surgical morbidity.
Therefore, we undertook a review of clinical cases from our institution performed between September 2011 and October 2016 in an attempt to understand if sclerotherapy as a primary treatment, or for vertebral body ABCs that were surgically addressed but that then recurred, could be efficacious for the axial skeleton or whether sclerotherapy as a primary treatment could help avoid surgical spinal intervention.
Non-surgical treatment of spinal ABCs is of significant interest. Interventional techniques for the treatment of ABCs throughout the body have grown in popularity over the past decade. Doxycycline has been used with excellent response in ABCs, with <12% recurrence rate.8 There can be significant surgical morbidity and it is difficult to fully eradicate all of the cysts. Additionally, surgical fixation hardware can make post-operative evaluation of the spine more difficult due to hardware artifacts. However, the use of non-surgical techniques often requires time to demonstrate bony healing.
Other groups have also performed minimally invasive therapies for ABCs. These include transoral vertebroplasty for a C2 lesion,10 chemotherapy, thermal ablation and selective arterial embolization.11–13 There is a single case report that also describes the use of doxycycline for treatment of a recurrent C2/C3 ABC.14 The use of doxycycline/albumin has also been reported by another team.15
Our review suggests that with close multidisciplinary follow-up, patients with spinal ABC can benefit from sclerotherapy and improve their presenting symptoms, or at minimum achieve stability of their presenting symptoms. Our sclerotherapy treatment regimen centered on the use of STS and doxycycline. Of particular note, treatment was only performed if the patient had symptomatic complaints or had a negative change in vertebral body height, suggesting progressive spinal instability.
We found that >50% of our cohort experienced stabilization of their symptoms or improvement, and we thus believe that, from the perspective of symptom stabilization, appropriately selected patients can be treated with sclerotherapy. This was not a foregone conclusion in this cohort, as the weight-bearing function of the spine is critically important and, as one of our patients demonstrated, failure to halt progression of disease can have fatal consequences. Therefore, our approach mandated that if spinal stability could not be achieved by sclerotherapy alone, surgical intervention must occur.
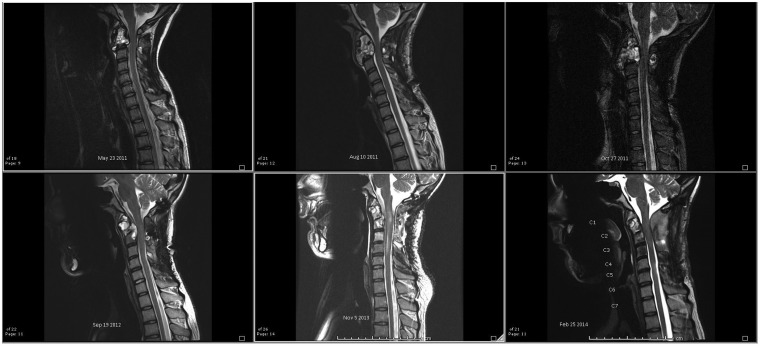
The one death merits further discussion. The patient received radiation at an outside institution prior to transferring care to ours. Initially at our institution, the patient was treated with transarterial Onyx embolization for a C2 ABC. Subsequently, the patient returned to the interventional radiology department after a C1–C3 occiput fusion with residual disease. Sclerotherapy was performed. The patient then returned 1 month and 3 months later for additional sclerotherapy. His symptoms were severe and unremitting (pain in the neck and arms), and therefore he underwent salvage occiput to C5 fusion. He had relief at this point, albeit temporary, with severe recurrence approximately 2 years later. Based on imaging undertaken when his symptoms were full-blown, there was a question of malignant transformation at the radiation site. The patient expired 1 month after the last clinical evaluation, where a multidisciplinary review of his options was undertaken and discussed with the family. After this review, the family elected to enter a home hospice program. Progression of disease could be seen on serial sagittal MRIs obtained over the course of his care (Figure 4).
Figure 4.
Progression of cervical spinal disease from presentation to prior to death; one angiographic embolization, two spinal fixations and two sclerotherapies were attempted to slow progression of disease, albeit without success.
There are aspects of this study that need to be addressed. Over the duration of this review, the technique and the agents utilized were modified. As such, while we did favor the use of STS with or without added doxycycline early in the study time frame, we primarily favored the use of doxycycline as a single treatment agent after 2014. The initial use of doxycycline and STS reflected an early approach to the treatment of ABCs. This transition came about as patient and outside scientific data were available for review and the assessment of outcomes. Understandably, our approach lends itself to difficulty in analyzing the data and our outcomes are somewhat difficult to quantify.
Additionally, our data are retrospective and lack significant quantification. There are various ways to quantify sclerotherapy response within bone, but the spine does not lend itself to easy quantification, particularly following surgical fixation. The use of cortical thickness or vertebral body height is challenged by artifacts from interval patient growth, or surgical stabilization in that clinical setting. Intralesional fibrosis or cortical thickness may offer a quantifiable endpoint, but this will also be subject to post-operative artifacts when applicable. These endpoints are also potentially subject to interpreter bias as there is not currently an accepted quantitative measure available. We also noted that there were instances of treatment response with persistent loss of vertebral body height, suggesting that fibrosis is not a stand-alone factor in spinal ABC stabilization. As we only treated symptomatic patients, one might consider a more aggressive approach to stabilization, such as continuing treatment empirically or on a defined protocol until robust evidence of healing is noted. Lastly, relative weaknesses of this work include a single operator for sclerotherapy and single-institution review.
With our early experience noted, a prospective database with symptom scoring is being constructed in order to better gauge clinical progress. Additionally, the optimal evaluation of spinal ABC patients may necessitate a multidisciplinary clinic where all specialists are present to formulate a robust treatment plan between the patient, surgeons and interventionalists. Surgical intervention might be necessitated sooner rather than later to maintain spinal stability and criteria should be better defined with respect to when surgical intervention should occur, with interventionalists playing a role with primary treatment or post-operative recurrence. Finally, we continue to review potential imaging endpoints in order to determine which may serve as the best indicators of disease/clinical stability.
Acknowledgements
The authors wish to thank Cindy Dube for her assistance with manuscript preparation and submission.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1.Biesecker JL, Marcove RC, Huvos AG, et al. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer 1970; 26: 615–625. [DOI] [PubMed] [Google Scholar]
- 2.Oliveira AM, His B-L, Weremowicz S, et al. USP6 (Tre2) fusion oncogenes in aneurysmal bone cyst. Cancer Res 2004; 64: 1920–1923. [DOI] [PubMed] [Google Scholar]
- 3.Capanna R, Campanacci DA, Manfrini M. Unicameral and aneurysmal bone cysts. Orthop Clin North Am 1996; 27: 605–614. [PubMed] [Google Scholar]
- 4.Gibbs CP, Jr, Hefele MC, Peabody TD, et al. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J Bone Joint Surg Am 1999; 81: 1671–1678. [DOI] [PubMed] [Google Scholar]
- 5.Bollini G, Jouve JL, Cottalorda J, et al. Aneurysmal bone cyst in children: Analysis of twenty-seven patients. J Pediatr Orthop B 1998; 7: 274–285. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh R, Alomari AI, Chaudry G, et al. Outcome of endovascular treatment for aneurysmal bone cysts. J Vasc Int Rad 2013; 24: S31. [Google Scholar]
- 7.Brosjö O, Pechon P, Hesla A, et al. Sclerotherapy with polidocanol for treatment of aneurysmal bone cysts. Acta Orthop 2013; 84: 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shiels WE, Mayerson J. Percutaneous doxycycline treatment of aneurysmal bone cysts with low recurrence rate: A preliminary report. Clin Orthop Relat Res 2013; 471: 2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Amer Acad Ortho Surg 2012; 20: 233–241. [DOI] [PubMed] [Google Scholar]
- 10.Brage L, Roldán H, Plata-Bello J, et al. Transoral vertebroplasty for a C2 aneurysmal bone cyst. Spine J 2016; 16: e473–e477. [DOI] [PubMed] [Google Scholar]
- 11.Charest-Morin R, Boriani S, Fisher CG, et al. Benign tumors of the spine: Has new chemotherapy and interventional radiology changed the treatment paradigm? Spine 2016; 41: S178–S185. [DOI] [PubMed] [Google Scholar]
- 12.Skubitz KM, Peltola JC, Santos ER, et al. Response of aneurysmal bone cyst to denosumab. Spine 2015; 40: E1201–E1204. [DOI] [PubMed] [Google Scholar]
- 13.Tobias L, Stehling C, Fröhlich B, et al. Denosumab: A potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J 2013; 22: 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle A. Recurrent aneurysmal bone cyst of the cervical spine in childhood treated with doxycycline injection. Skeletal Radiol 2015; 44: 609–612. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Han SB, Yang SM, et al. Percutaneous albumin/doxycycline injection versus open surgery for aneurysmal bone cysts in the mobile spine. Eur Spine J. Epub ahead of print 23 November 2018. DOI: 10.1007/s00586-018-5836-1. [DOI] [PubMed] [Google Scholar]