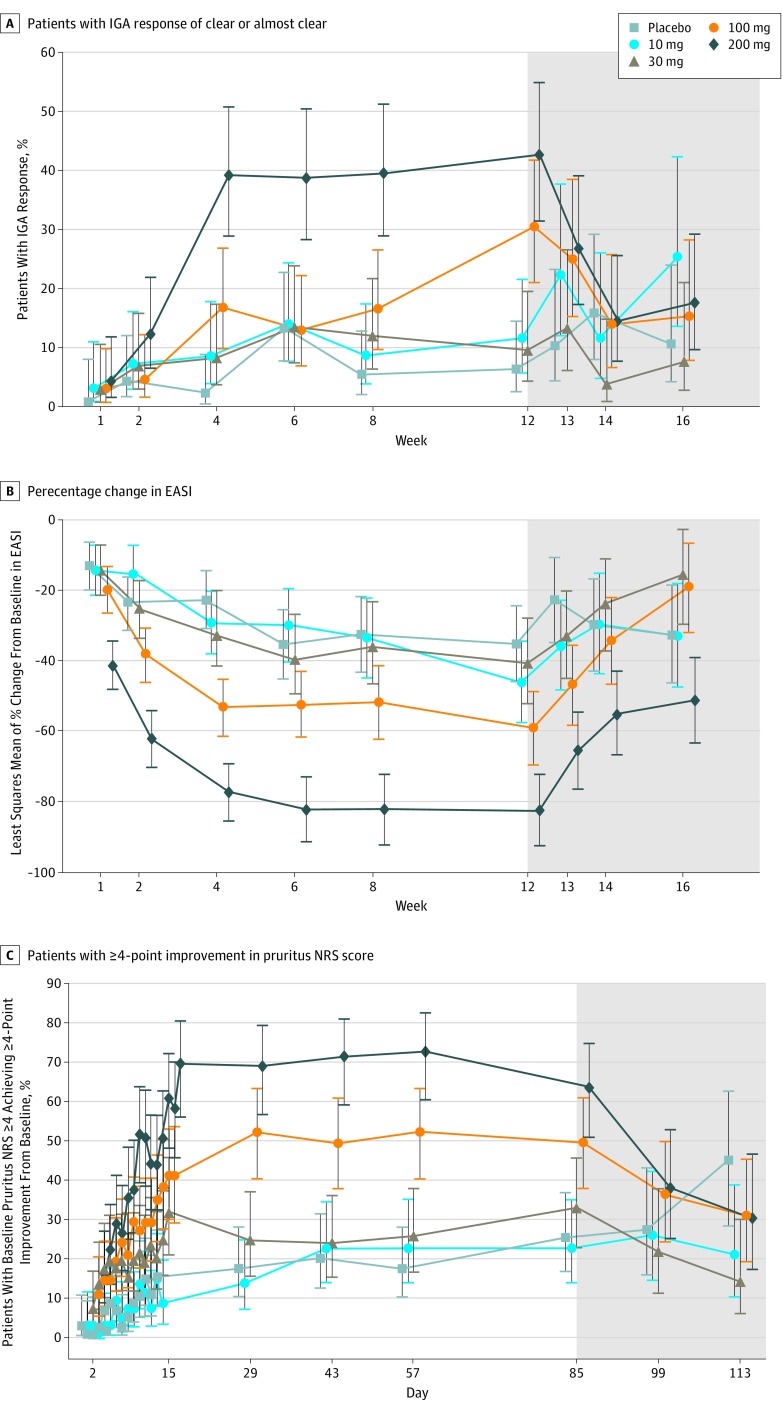
Figure 2. Secondary Efficacy End Points.
A, Proportion of patients who achieved Investigator’s Global Assessment (IGA) of clear or almost clear with 2-grade or more improvement from baseline over time. A logistic regression model was used, including treatment as a main effect, baseline IGA as a covariate. B, Percentage change from baseline in Eczema Area and Severity Index (EASI) over time. C, Proportion of patients with baseline pruritus numeric rating scale (NRS) score of 4 or higher, achieving 4-point or more improvement from baseline over time. Mixed-effects model repeated measure was used and contained fixed factors of treatment, week, treatment by week interaction, baseline value, and unstructured covariance matrix. Error bars denote 90% confidence interval. Missing data were not imputed. Baseline was defined as the last measurement before first dosing. Shaded areas represent the follow-up period when patients were no longer receiving the drug.