Abstract
Objective
Choosing therapies for type 2 diabetes that are both effective and cost-effective is vital as healthcare systems worldwide aim to maximize health of the population. The present analysis assessed the cost-effectiveness of once-weekly semaglutide (a novel glucagon-like peptide-1 (GLP-1) receptor agonist) versus insulin glargine U100 (the most commonly used basal insulin) and versus dulaglutide (an alternative once-weekly GLP-1 receptor agonist), from a societal perspective in the Netherlands.
Research design and methods
The IQVIA CORE Diabetes Model was used to project outcomes for once-weekly semaglutide 0.5 mg and 1 mg versus insulin glargine U100, once-weekly semaglutide 0.5 mg versus dulaglutide 0.75 mg, and once-weekly semaglutide 1 mg versus dulaglutide 1.5 mg. Clinical data were taken from the SUSTAIN 4 and SUSTAIN 7 clinical trials. The analysis captured direct and indirect costs, mortality, and the impact of diabetes-related complications on quality of life.
Results
Projections of outcomes suggested that once-weekly semaglutide 0.5 mg was associated with improved quality-adjusted life expectancy by 0.19 quality-adjusted life years (QALYs) versus insulin glargine U100 and 0.07 QALYs versus dulaglutide 0.75 mg. Once-weekly semaglutide 1 mg was associated with mean increases in quality-adjusted life expectancy of 0.27 QALYs versus insulin glargine U100 and 0.13 QALYs versus dulaglutide 1.5 mg. Improvements came at an increased cost versus insulin glargine U100, with incremental cost-effectiveness ratios from a societal perspective of €4988 and €495 per QALY gained for once-weekly semaglutide 0.5 mg and 1 mg, respectively, falling below Netherlands-specific willingness-to-pay thresholds. Improvements versus dulaglutide came at a reduced cost from a societal perspective for both doses of once-weekly semaglutide.
Conclusions
Once-weekly semaglutide is cost-effective versus insulin glargine U100, and dominant versus dulaglutide 0.75 and 1.5 mg for the treatment of type 2 diabetes, and represents a good use of healthcare resources in the Netherlands.
Keywords: non-insulin dependent diabetes mellitus, glucagon-like peptide-1 (GLP-1), cost effectiveness, economics/cost
Significance of this study.
What is already known about this subject?
Choosing therapies for type 2 diabetes that are both effective and cost-effective is vital as healthcare systems worldwide aim to maximize health of the population, and the WHO recommends the use of cost-effectiveness analysis to ensure that interventions funded by a healthcare system represent good value for money. The cost-effectiveness of once-weekly semaglutide, a novel glucagon-like peptide-1 receptor agonist, has been assessed in a number of countries, but numerous factors may influence the cost-effectiveness of interventions in different settings. The aim of the present analysis was to assess the cost-effectiveness of once-weekly semaglutide from a societal perspective in the Netherlands.
What are the new findings?
Projections of outcomes over patient lifetimes suggest that once-weekly semaglutide 0.5 mg and 1 mg are likely to improve clinical outcomes for patients with type 2 diabetes compared with insulin glargine U100 and dulaglutide. Compared with insulin glargine U100, improvements in clinical outcomes came at an increased cost, but once-weekly semaglutide was considered cost-effective, even at the lowest willingness-to-pay threshold identified in the Netherlands. Improvements came at a reduced cost from a societal perspective versus dulaglutide, and therefore once-weekly semaglutide was considered dominant.
How might these results change the focus of research or clinical practice?
Use of once-weekly semaglutide for treatment of patients with type 2 diabetes is likely to be a good use of healthcare resources in the Netherlands.
Introduction
Choosing therapies to treat people with type 2 diabetes that are both effective and cost-effective is vital as healthcare systems worldwide aim to maximize health of the population while operating under resource constraints. Finite resources allocated to healthcare are coming under increasing pressure due to both growing demand and limited budget increases. The WHO recommends the use of cost-effectiveness analysis to ensure that funded interventions represent good value for money.1 This is particularly pertinent when considering type 2 diabetes, where the number of people with diabetes worldwide is expected to increase from 424.9 million in 2017 to 628.6 million in 2045, with total healthcare expenditure due to diabetes expected to rise from US$850 billion to US$958 billion over the same period.2 Diabetes-related complications have a significant impact on the health status of people with diabetes, and are associated with significant costs, with annual costs increasing 1.7-fold, 2-fold and 3.5-fold in people with microvascular, macrovascular, and both microvascular and macrovascular complications, respectively, compared with people with diabetes with no complications.3 Therefore, reducing the frequency of diabetes-related complications by controlling risk factors, including glycemia, blood pressure, and body weight, is key for effective and cost-effective therapies.4–7
Glucagon-like peptide-1 (GLP-1) receptor agonists are a modern therapy for type 2 diabetes with multifactorial benefits, reducing glycated hemoglobin (HbA1c) and body weight with a low risk of hypoglycemia.8 The most recent consensus statement released by the European Association for the Study of Diabetes and the American Diabetes Association states that GLP-1 receptor agonists should be considered for people with type 2 diabetes not achieving glycemic control targets on metformin, particularly those with established cardiovascular disease (where evidence for a cardioprotective effect is strongest with liraglutide and semaglutide), with a compelling need to minimize hypoglycemia, or with a compelling need to minimize weight gain or promote weight loss.9 In the Netherlands, GLP-1 receptor agonists are recommended for patients with a body mass index (BMI) ≥35 kg/m2 whose blood glucose values cannot be adequately regulated with the combination of metformin and a sulfonylurea, and those with a BMI≥30 kg/m2 who do not achieve blood glucose targets with optimally titrated basal insulin in combination with metformin (with or without sulfonylurea).10
Diabetes mellitus is a common chronic disease in the Netherlands, with over 1.1 million people affected by the condition.11 Estimates suggest that the total economic burden associated with diabetes in 2016 was €6.8 billion.12 Of this, €2.8 billion was associated with direct healthcare costs, of which €1.3 billion was related to costs of complications, and €4 billion was associated with indirect costs, such as lost productivity, welfare payments, and indirect costs of complications.12 Therefore, prescribing cost-effective therapies for people with diabetes represents an opportunity to optimize the use of resources within the Dutch healthcare system. The present analysis assessed the cost-effectiveness of once-weekly semaglutide, a novel GLP-1 receptor agonist, from a societal perspective in the Netherlands. Once-weekly semaglutide was compared with once-daily insulin glargine U100 (the most commonly used basal insulin in the Netherlands) and once-weekly dulaglutide, an alternative once-weekly GLP-1 receptor agonist.
Methods
Approach
A cost-effectiveness analysis was performed by projecting costs and clinical outcomes over patient lifetimes following initiation of treatment with once-weekly semaglutide, daily insulin glargine U100, or dulaglutide. This approach aims to capture all complications, and therefore their impact on costs, life expectancy, and quality of life, as is recommended in guidelines on assessing the cost-effectiveness of interventions for diabetes.13 The analysis was performed using the IQVIA CORE Diabetes Model, a non-product-specific computer simulation model of diabetes, the capabilities and features of which have been published previously.14 Long-term outcomes projected by the model were validated against long-term clinical data on first publication of the model in 2004 and following a series of model updates in 2014.15 16
Modeled outcomes included direct medical costs, indirect costs, life expectancy (measured in years), quality-adjusted life expectancy (measured in quality-adjusted life years (QALYs)), and the cumulative incidence and time to onset of diabetes-related complications. In cases where an intervention is associated with increased costs and greater clinical benefits, costs and effectiveness are combined to give an incremental cost-effectiveness ratio (ICER), describing the incremental cost per unit of effectiveness gained for the tested intervention versus the comparator, allowing assessment of whether an intervention represents good value for money. In scenarios where an intervention is associated with reduced costs and increased clinical benefits, it is considered dominant versus the comparator and no calculation of an ICER is required. The modeling analysis took into account mortality following diabetes-related complications, and background mortality based on Netherlands-specific life tables.17 Future clinical benefits were discounted at 1.5% per annum and future costs were discounted at 4% per annum, in line with guidelines for economic evaluation in the Netherlands.18
Clinical data
Clinical data for the comparison of once-weekly semaglutide with daily insulin glargine U100 were taken from the SUSTAIN 4 clinical trial, while the SUSTAIN 7 clinical trial provided data for the comparison with dulaglutide.19 20 SUSTAIN 4 was a 30-week open-label trial comparing once-weekly semaglutide 0.5 mg and 1 mg with daily insulin glargine U100 in people with type 2 diabetes with an HbA1c of 7.0%–10.0% on metformin either alone or in combination with a sulfonylurea. SUSTAIN 7 was a 40-week, randomized, open-label trial comparing once-weekly semaglutide 0.5 mg and 1 mg with once-weekly dulaglutide 0.75 and 1.5 mg in people with type 2 diabetes with an HbA1c of 7.0%–10.5% while on metformin monotherapy. Data were obtained from prespecified endpoints wherever possible, but in order to fulfill all of the data requirements for an analysis using the IQVIA CORE Diabetes Model, a number of post hoc data extractions from the trial data were required (serum lipids and hypoglycemic events, to ensure that definitions met those used in the IQVIA CORE Diabetes Model).
The cost-effectiveness analysis used the baseline cohorts from SUSTAIN 4 and 7 (table 1) and applied the changes from baseline and adverse event rates from the end of the respective trials (table 2). The clinical benefits, adverse events and costs associated with rescue therapy were not captured in the analysis. In line with the trial protocols, both doses of once-weekly semaglutide were compared with insulin glargine, once-weekly semaglutide 0.5 mg was compared with dulaglutide 0.75 mg, and once-weekly semaglutide 1 mg was compared with dulaglutide 1.5 mg. Across all comparisons, once-weekly semaglutide was associated with statistically significant improvements in HbA1c and BMI versus the comparator therapy.
Table 1.
Baseline cohort characteristics
| SUSTAIN 4 | SUSTAIN 7 | |
| Age (years) | 56 (10) | 56 (11) |
| Duration of diabetes (years) | 9 (6)* | 7 (6)* |
| Percentage male (%) | 53 | 55 |
| HbA1c (%) | 8.3 (0.9) | 8.2 (0.9) |
| Systolic blood pressure (mm Hg) | 132 (15) | 133 (14) |
| Total cholesterol (mg/dL) | 179 (42) | 181 (43) |
| HDL cholesterol (mg/dL) | 46 (12) | 45 (11) |
| LDL cholesterol (mg/dL) | 97 (35) | 102 (37) |
| Triglycerides (mg/dL) | 190 (124) | 181 (109) |
| BMI (kg/m2) | 33.0 (6.5) | 33.5 (6.8) |
Values are means (SD).
*Rounded as the IQVIA CORE Diabetes Model only accepts integer values for duration of diabetes.
BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein;LDL, low-density lipoprotein.
Table 2.
Treatment effects and adverse event rates
| SUSTAIN 4 | SUSTAIN 7 | ||||||
| Semaglutide 0.5 mg | Semaglutide 1 mg | Insulin glargine U100 | Semaglutide 0.5 mg | Dulaglutide 0.75 mg | Semaglutide 1 mg | Dulaglutide 1.5 mg | |
| HbA1c (%) | −1.2 (0.1)* | −1.6 (0.1)* | −0.8 (0.1) | −1.5 (0.1)* | −1.1 (0.1) | −1.8 (0.1)* | −1.4 (0.1) |
| Systolic blood pressure (mm Hg) | −5 (0.7)* | −5 (0.7)* | −2 (0.7) | −2 (0.8) | −2 (0.8) | −5 (0.8) | −3 (0.8) |
| Total cholesterol (mg/dL) | −9 (1.6)* | −9 (1.6)* | −2 (1.6) | −7 (1.7) | −6 (1.8) | −5 (1.8) | −3 (1.8) |
| HDL cholesterol (mg/dL) | −1 (0.4) | 0 (0.4)* | −1 (0.3) | 0 (0.3) | 0 (0.4) | 1 (0.4) | 1 (0.4) |
| LDL cholesterol (mg/dL) | −4 (1.3)* | −5 (1.3)* | 1 (1.4) | −3 (1.5) | −3 (1.5) | 0 (1.6) | 1 (1.5) |
| Triglycerides (mg/dL) | −18 (3.2) | −22 (3.1)* | −12 (3.2) | −14 (3.1) | −14 (3.1) | −22 (2.9) | −16 (3.0) |
| BMI (kg/m2) | −1.2 (0.1)* | −1.9 (0.1)* | 0.4 (0.1) | −1.6 (0.1)* | −0.8 (0.1) | −2.3 (0.1)* | −1.1 (0.1) |
| Non-severe hypoglycemia (events per 100 patient-years) | 12 | 13 | 27 | 1 | 1 | 3 | 1 |
| Severe hypoglycemia (events per 100 patient-years) | 2 | 5 | 2 | 0 | 0 | 0 | 1 |
| Proportion of non-severe events that are nocturnal | 0.18 | 0.11 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 |
| Proportion of severe events that are nocturnal | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 |
*Statistically significant difference at 95% confidence level. Values are means (SE).
BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Patients were assumed to receive once-weekly semaglutide, dulaglutide or insulin glargine U100 29.2 IU (the end of trial dose in SUSTAIN 4) for the first 3 years of the analysis, based on a review of treatments for type 2 diabetes conducted in 2017, which concluded that the mean duration of GLP-1 receptor agonist treatment was 29.4 months, as patients may switch to more advanced therapy due to the progressive nature of the disease.21 This was rounded up to 3 years, as treatment switching is only possible at the end of a given year in the IQVIA CORE Diabetes Model. After 3 years, once-weekly semaglutide or dulaglutide treatment was discontinued and patients received 40 IU insulin glargine U100 per day. Patients in the insulin glargine arm continued this therapy, with the dose increased from 29.2 to 40 IU. This resulted in equal treatment costs in all arms following treatment intensification at 3 years. This approach is in line with previous long-term cost-effectiveness analyses of GLP-1 receptor agonists submitted to the Scottish Medicines Consortium and the National Institute for Health and Care Excellence, and published in peer-reviewed journals.22–27 Differences in HbA1c, BMI and hypoglycemic event rates between the study arms were assumed to persist for the first 3 years of the analysis, as patients received different therapies and accrued different treatment costs in this period. This resulted in a balanced cost-effectiveness analysis, with differences in these key inputs maintained only while there were differences in treatment costs.
Costs
The analysis evaluated cost-effectiveness from a societal perspective, capturing both direct and indirect costs in 2017 euros (EUR), in line with guidance on the evaluation of new health technologies in the Netherlands.18 Direct costs included costs of diabetes medications, costs of consumables (such as needles for injection of insulin and self-monitoring of blood glucose supplies), and costs associated with diabetes-related complications. Indirect costs were assessed in terms of lost workplace productivity due to diabetes-related complications and mortality.
Pharmacy and consumables costs were based on list prices published for the Netherlands (online supplementary table 1).28 Diabetes medication resource use was taken from the SUSTAIN 4 and 7 trials, and used to calculate annual treatment costs. A mean daily dose of 29.2 IU insulin glargine U100 was used to calculate annual treatment costs over the first 3 years of the analysis in the insulin glargine arm, based on the end of trial dose in SUSTAIN 4 (no increase in insulin dose over the 3 years was modeled). After 3 years, a dose of 40 IU insulin glargine U100 was used to calculate treatment costs in all treatment arms. A targeted literature review was performed in 2018 to collect costs associated with diabetes-related complications in the Netherlands (online supplementary table 2). Indirect costs were based on days off work estimates following diabetes-related complications, and mean salary and retirement age in the Netherlands.29
bmjdrc-2019-000705supp001.pdf (1.5MB, pdf)
Quality of life utilities
As diabetes progresses, patients develop complications that influence their overall health-related quality of life. It was therefore important to evaluate utility levels associated with each of the complications modeled. Utilities relating to quality of life were taken from a 2014 review by Beaudet et al with the exception of hypoglycemic event disutilities, which were sourced from a 2013 publication by Evans et al (published after the literature searches by Beaudet et al had been conducted).30 31
Sensitivity analyses
Projection of outcomes over patient lifetimes based on short-term clinical trial data is associated with uncertainty, and therefore sensitivity analyses were conducted to evaluate the robustness of the results and identify key drivers of modeled outcomes. Sensitivity analyses included variation of the time horizon of the analysis, discount rates applied, treatment effects applied, HbA1c progression approaches, timing of treatment switching, costs of complications, risk equations used to predict cardiovascular events, and utilities applied. In addition, probabilistic sensitivity analysis (PSA) was performed using the predefined function in the IQVIA CORE Diabetes Model.
Subgroup analyses
The base case analyses were based on data from all patients enrolled in the SUSTAIN 4 and 7 clinical trials, in order to use the most robust data sources to inform the analyses. However, in the Netherlands, the reimbursement of GLP-1 receptor agonists is currently limited to people with type 2 diabetes with a BMI≥35 kg/m2 whose blood glucose values cannot be adequately regulated with the combination of metformin and a sulfonylurea, and those with a BMI ≥30 kg/m2 who do not achieve blood glucose targets with optimally titrated basal insulin in combination with metformin (with or without sulfonylurea).10 Therefore, subgroup analyses based on patients with BMI ≥30 and ≥35 kg/m2 at baseline in the SUSTAIN 4 and 7 trials were prepared.
Results
Once-weekly semaglutide versus once-daily insulin glargine
Base case analyses
Both doses of once-weekly semaglutide were associated with improved discounted life expectancy and quality-adjusted life expectancy versus once-daily insulin glargine U100 (table 3). Once-weekly semaglutide 0.5 mg was associated with improvements in life expectancy and quality-adjusted life expectancy of 0.20 years and 0.19 QALYs per patient, respectively, and benefits were slightly greater with once-weekly semaglutide 1 mg at 0.28 years and 0.27 QALYs per patient, respectively. A reduced incidence of diabetes-related complications and an increased time to their onset led to improved duration and quality of life with once-weekly semaglutide 0.5 mg and 1 mg compared with insulin glargine U100.
Table 3.
Base case results
| Semaglutide 0.5 mg | Insulin glargine U100 | Difference | |
| Discounted life expectancy (years) | 18.35 (0.27) | 18.15 (0.29) | +0.20 |
| Discounted quality-adjusted life expectancy (QALYs) | 12.05 (0.18) | 11.85 (0.19) | +0.19 |
| Discounted direct costs (€) | 26 780 (1054) | 24 627 (1136) | +2152 |
| Discounted combined costs (€) | 46 860 (1903) | 45 911 (1967) | +949 |
| ICER based on direct costs | €11 310 per QALY gained | ||
| ICER based on combined costs | €4988 per QALY gained | ||
| Semaglutide 1 mg | Insulin glargine U100 | Difference | |
| Discounted life expectancy (years) | 18.44 (0.27) | 18.15 (0.29) | +0.28 |
| Discounted quality-adjusted life expectancy (QALYs) | 12.12 (0.18) | 11.85 (0.19) | +0.27 |
| Discounted direct costs (€) | 26 654 (1112) | 24 627 (1136) | +2027 |
| Discounted combined costs (€) | 46 044 (1934) | 45 911 (1967) | +133 |
| ICER based on direct costs | €7515 per QALY gained | ||
| ICER based on combined costs | €495 per QALY gained | ||
| Once-weekly semaglutide 0.5 mg | Dulaglutide 0.75 mg | Difference | |
| Discounted life expectancy (years) | 17.56 (0.29) | 17.51 (0.27) | +0.06 |
| Discounted quality-adjusted life expectancy (QALYs) | 11.53 (0.19) | 11.46 (0.18) | +0.07 |
| Discounted direct costs (€) | 26 133 (1070) | 25 819 (1124) | +314 |
| Discounted combined costs (€) | 46 160 (1935) | 46 606 (2013) | −446 |
| ICER based on direct costs | €4671 per QALY gained | ||
| ICER based on combined costs | Once-weekly semaglutide 0.5 mg dominant | ||
| Semaglutide 1 mg | Dulaglutide 1.5 mg | Difference | |
| Discounted life expectancy (years) | 17.69 (0.27) | 17.55 (0.28) | +0.14 |
| Discounted quality-adjusted life expectancy (QALYs) | 11.63 (0.18) | 11.50 (0.18) | +0.13 |
| Discounted direct costs (€) | 25 945 (1025) | 25 565 (1070) | +381 |
| Discounted combined costs (€) | 45 365 (1880) | 45 820 (1922) | −455 |
| ICER based on direct costs | €2861 per QALY gained | ||
| ICER based on combined costs | Once-weekly semaglutide 1 mg dominant | ||
Values are means (SD).
€, 2017 euros (EUR); ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
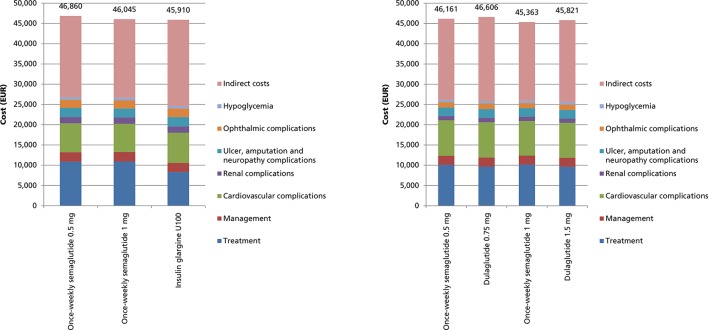
Both doses of once-weekly semaglutide were associated with increased direct costs compared with insulin glargine U100. This resulted from higher pharmacy costs during the first 3 years of the analysis (figure 1), as once-weekly semaglutide 0.5 mg and 1 mg were associated with higher treatment costs than 29.2 IU insulin glargine U100 once daily (based on the end of study dose). Reduced costs of treating diabetes-related complications partially offset higher pharmacy costs, with the largest cost savings resulting from avoided cardiovascular complications (mean cost savings of €283 and €425 per patient with once-weekly semaglutide 0.5 mg and 1 mg, respectively). Both doses of once-weekly semaglutide were associated with fewer diabetes-related complications, leading to less lost workplace productivity and mean indirect cost savings of €1203 with once-weekly semaglutide 0.5 mg and €1893 with once-weekly semaglutide 1 mg. When direct and indirect costs were combined, once-weekly semaglutide 0.5 mg was associated with increased costs of €949 per patient and once-weekly semaglutide 1 mg was associated with increased costs of €133 per patient versus insulin glargine U100.
Figure 1.
Costs over patient lifetimes in the base case analyses.
Projections over patient lifetimes suggested that once-weekly semaglutide 0.5 mg was associated with ICERs of €11 310 per QALY gained based on direct costs, and €4988 per QALY gained based on combined costs versus insulin glargine U100. Once-weekly semaglutide 1 mg was associated with ICERs based on direct and combined costs of €7515 per QALY gained and €495 per QALY gained versus insulin glargine U100, respectively.
Sensitivity analyses
The conclusions of the base case analyses were confirmed by extensive sensitivity analyses. Full results of the sensitivity analyses comparing once-weekly semaglutide 0.5 mg and 1 mg with insulin glargine U100 can be found in the online supplementary tables 3 and 4. Cost-effectiveness scatterplots and cost-effectiveness acceptability curves generated in the PSA are also available in the online supplementary figures 1 and 2.
Subgroup analyses
Projection of long-term clinical outcomes in people with diabetes with BMI ≥30 and ≥35 kg/m2 showed that results were consistent with the base case analysis in all patients (table 4). Results were similar in all three analyses comparing once-weekly semaglutide 0.5 mg with insulin glargine U100, with comparable differences in life expectancy, quality-adjusted life expectancy, direct costs, and indirect costs. The 1 mg dose of once-weekly semaglutide was associated with greater clinical benefits over insulin glargine U100 in people with a higher BMI at baseline. ICERs based on direct costs fell to €6384 per QALY gained in people with a BMI≥30 kg/m2 and €5564 per QALY gained in people with a BMI≥35 kg/m2. When combined costs were taken into account, once-weekly semaglutide 1 mg was found to be dominant versus insulin glargine U100 in people with BMI ≥30 and ≥35 kg/m2.
Table 4.
Subgroup analysis results
| Base case (all patients) | BMI≥30 kg/m2 | BMI≥35 kg/m2 | |
| SUSTAIN 4: Semaglutide 0.5 mg versus insulin glargine U100 | |||
| ICER based on direct costs | €11 310 per QALY gained | €11 184 per QALY gained | €13 205 per QALY gained |
| ICER based on combined costs | €4988 per QALY gained | €4541 per QALY gained | €7463 per QALY gained |
| SUSTAIN 4: Semaglutide 1 mg versus insulin glargine U100 | |||
| ICER based on direct costs | €7515 per QALY gained | €6384 per QALY gained | €5564 per QALY gained |
| ICER based on combined costs | €495 per QALY gained | Semaglutide dominant | Semaglutide dominant |
| SUSTAIN 7: Semaglutide 0.5 mg versus dulaglutide 0.75 mg | |||
| ICER based on direct costs | €4671 per QALY gained | €3917 per QALY gained | €2149 per QALY gained |
| ICER based on combined costs | Semaglutide dominant | Semaglutide dominant | Semaglutide dominant |
| SUSTAIN 7: Semaglutide 1 mg versus dulaglutide 1.5 mg | |||
| ICER based on direct costs | €2861 per QALY gained | €2855 per QALY gained | €3392 per QALY gained |
| ICER based on combined costs | Semaglutide dominant | Semaglutide dominant | Semaglutide dominant |
€, 2017 euros (EUR); BMI, body mass index; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Once-weekly semaglutide versus dulaglutide
Base case analyses
Projections of outcomes over patient lifetimes suggested that once-weekly semaglutide 0.5 mg was associated with improved discounted life expectancy and quality-adjusted life expectancy by 0.06 years and 0.07 QALYs per patient, respectively, versus dulaglutide 0.75 mg (table 3). Similarly, once-weekly semaglutide 1 mg was associated with mean increases in life expectancy of 0.14 years and quality-adjusted life expectancy of 0.13 QALYs versus dulaglutide 1.5 mg (table 3). In both cases, improvements in clinical outcomes were driven by a reduced cumulative incidence and delayed time to onset of complications over the long term.
When direct costs were considered, both 0.5 mg and 1 mg doses of once-weekly semaglutide were associated with increased treatment costs compared with dulaglutide 0.75 and 1.5 mg, due to higher pharmacy costs over the first 3 years of the analysis (figure 1). However, this was partially offset by cost savings resulting from avoided complications, most notably ophthalmic complications with once-weekly semaglutide 0.5 mg and cardiovascular complications with once-weekly semaglutide 1 mg. Once-weekly semaglutide 0.5 mg and 1 mg were associated with mean indirect cost savings of €760 and €835 per patient, respectively, resulting from reduced lost productivity due to fewer diabetes-related complications and reduced mortality. ICERs based on direct costs were €4671 per QALY gained for once-weekly semaglutide 0.5 mg versus dulaglutide 0.75 mg, and €2861 per QALY gained for once-weekly semaglutide 1 mg versus dulaglutide 1.5 mg. When combined (both direct and indirect) costs were included, both doses of once-weekly semaglutide were found to be dominant (associated with improved clinical outcomes and cost savings) versus the comparator doses of dulaglutide.
Sensitivity analyses
Extensive sensitivity analyses confirmed the conclusions of the base case analyses. Full results of the sensitivity analyses comparing once-weekly semaglutide 0.5 mg with dulaglutide 0.75 mg, and once-weekly semaglutide 1 mg with dulaglutide 1.5 mg can be found in the online supplementary tables 5 and 6. Cost-effectiveness scatterplots and cost-effectiveness acceptability curves generated in the PSA are also available in the online supplementary figures 3–6.
Subgroup analyses
Evaluation of outcomes in people with diabetes with BMI ≥30 and ≥35 kg/m2 found that the conclusions did not differ from the base case analyses conducted in all patients (table 4). Differences in life expectancy, quality-adjusted life expectancy, direct costs and indirect costs were similar to the base case analyses. Once-weekly semaglutide 0.5 mg remained dominant versus dulaglutide 0.75 mg and once-weekly semaglutide 1 mg was found to be dominant versus dulaglutide 1.5 mg in both subgroups.
Discussion
Projections over patient lifetimes based on two randomized controlled trials suggested that once-weekly semaglutide would improve clinical outcomes compared with insulin glargine U100 and dulaglutide for treatment of people with type 2 diabetes. These improvements were achieved at an increased cost from a societal perspective versus insulin glargine U100 but at a reduced cost from a societal perspective versus dulaglutide. Decision-making around reimbursement of interventions which improve outcomes and reduce costs is straightforward, but decision-making when interventions improve outcomes and increase costs is more nuanced, requiring assessment of whether the additional benefits are worth the additional costs. These value-for-money assessments can be made by comparing the ICER generated in the analysis with a willingness-to-pay threshold, representing the maximum amount an individual or society is prepared to pay in order to gain 1 QALY. A number of willingness-to-pay thresholds have been suggested for the Netherlands, with €20 000 per QALY gained often quoted, and discussion of a willingness-to-pay threshold of €80 000 per QALY gained for very severe diseases.32 A study attempting to identify the willingness-to-pay threshold of individuals in the Netherlands found that the willingness-to-pay threshold ranged from €12 900 per QALY gained to €24 500 per QALY gained, depending on the measure used to assess quality of life.33 All base case ICERs versus insulin glargine U100 and dulaglutide in the present analysis fell below the lowest willingness-to-pay threshold, even with the most stringent definition of value for money in the Netherlands. Use of once-weekly semaglutide for treatment of patients with type 2 diabetes in the Netherlands is, therefore, likely to be a good use of healthcare resources.
At present, reimbursement of GLP-1 receptor agonists in the Netherlands is limited to people with type 2 diabetes with a BMI≥35 kg/m2 whose blood glucose values cannot be adequately regulated with the combination of metformin and a sulfonylurea, and those with a BMI≥30 kg/m2 who do not achieve blood glucose targets with optimally titrated basal insulin in combination with metformin (with or without a sulfonylurea).10 The present analysis assessed outcomes in all patients in the base case analyses, in line with the inclusion criteria of the SUSTAIN 4 and 7 clinical trials. In the SUSTAIN 4 and 7 trials, mean BMI was 33.01 and 33.50 kg/m2, respectively, and therefore a number of patients would meet the reimbursement criteria for a GLP-1 receptor agonist, but others would not. To investigate the impact of baseline BMI on cost-effectiveness, the present analysis included subgroup analyses in patients with BMI ≥30 and ≥35 kg/m2. These analyses showed that once-weekly semaglutide is likely to be cost-effective in all patient groups analyzed and therefore is likely to be a good use of healthcare resources in patients with a BMI≥30 kg/m2.
Cost-effectiveness analyses in other country settings support the results of the present analysis. An evaluation based on SUSTAIN 7 for the UK found that both doses of once-weekly semaglutide were dominant versus dulaglutide from a healthcare payer perspective.27 Similarly, in an analysis prepared for the Canadian setting (using a different health economic model), once-weekly semaglutide 0.5 mg was dominant versus dulaglutide 0.75 mg and once-weekly semaglutide 1 mg was dominant versus dulaglutide 1.5 mg.34 In Estonia, a cost-effectiveness analysis has suggested that once-weekly semaglutide is cost-effective versus liraglutide, improving clinical outcomes with only a small increase in costs.35 An analysis for Denmark found that once-weekly semaglutide 0.5 mg and 1 mg were either cost-effective or dominant versus a range of GLP-1 receptor agonists.36 Numerous factors may influence the cost-effectiveness of interventions in different countries, including pharmacy costs, costs of treating complications, and healthcare funding models, but once-weekly semaglutide appears to be consistently cost-effective in a range of countries. To date, no other studies have assessed the cost-effectiveness of once-weekly semaglutide versus insulin glargine U100 based on SUSTAIN 4.
There is increasing interest in the impact of GLP-1 receptor agonists on cardiovascular risk. Efficacy appears to vary between GLP-1 receptor agonists, with once-weekly semaglutide, liraglutide, dulaglutide and albiglutide shown to reduce the risk of cardiovascular events, but lixisenatide and exenatide extended release shown to have no impact compared with placebo.37–42 It was not possible to take into account the impact of interventions on cardiovascular risk in the present analysis, as risk equations based on the cardiovascular outcome studies have not yet been integrated into cost-effectiveness models. Exclusion of the direct cardioprotective effect observed with semaglutide, particularly in the comparison with insulin glargine U100 which has been shown to have no cardioprotective effect, is a conservative approach, with a modeling study based on the SUSTAIN 6 trial showing that conventional risk equations underestimate the reduced incidence of stroke with once-weekly semaglutide.43 The cardiovascular outcome trials in type 2 diabetes have been conducted in patients with varying characteristics, most notably with differing cardiovascular risk profiles at baseline. Studies in homogeneous populations are required to assess the relative impact of different GLP-1 receptor agonists on cardiovascular risk, and subsequently capture any differences in risk equations for use in health economic analyses. Nevertheless, the cardiovascular benefits for semaglutide reported in the SUSTAIN 6 study may increase the cost-effectiveness of once-weekly semaglutide, particularly in comparison with insulin glargine U100.
As with many health economic analyses of interventions for type 2 diabetes, a limitation of the present analysis was the use of short-term data to project long-term outcomes. However, modeled projections represent the best available option for healthcare decision-making in the absence of long-term clinical trial data. Projection of outcomes over patient lifetimes is recommended in guidelines for assessing the cost-effectiveness of diabetes interventions, and the present analysis attempted to mitigate the inherent uncertainty through use of a published and extensively validated model and through preparation of extensive sensitivity analyses.13–16
A further limitation of the analysis was that adherence and persistence were not captured. There are currently no data to inform rates of adherence and persistence with once-weekly semaglutide, dulaglutide and insulin glargine U100 over the long term. There are also no data to inform how risk factors, such as HbA1c and BMI would be affected by non-adherence and non-persistence. Similarly, switching between doses of GLP-1 receptor agonists and titration of the insulin glargine U100 was not captured, as there are no data available to inform rates of switching or titration, or the subsequent changes in risk factors. Further studies in real-world clinical practice are required to examine adherence, persistence and changes in dose, and how these affect risk factors for diabetes-related complications, with these data then included in further evaluations of cost-effectiveness.
Conclusions
Projections suggest that once-weekly semaglutide 0.5 mg and 1 mg are likely to improve clinical outcomes for patients with type 2 diabetes compared with insulin glargine and dulaglutide. Compared with insulin glargine U100, improvements in clinical outcomes came at an increased cost, but once-weekly semaglutide was considered cost-effective, even at the lowest willingness-to-pay threshold identified in the Netherlands. Improvements came at a reduced cost from a societal perspective versus dulaglutide, and therefore once-weekly semaglutide was considered dominant. Use of once-weekly semaglutide for treatment of patients with type 2 diabetes is, therefore, likely to be a good use of healthcare resources in the Netherlands.
Footnotes
Correction notice: This article has been corrected since it was published. Author name and affiliation has been updated.
Contributors: The study was conceived and designed by all authors and conducted by BH. BH drafted the manuscript, which was reviewed and revised by all authors. All authors had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: The study was supported by funding from Novo Nordisk Region Europe Pharmaceuticals A/S.
Competing interests: SJPM and BH are employees of Ossian Health Economics and Communications. Ossian received consulting fees from Novo Nordisk Region Europe Pharmaceuticals A/S to support preparation of the analysis. RGJM and ELH are employees of Novo Nordisk BV. TV is an employee of SA Novo Nordisk Pharma. BHRW has received grant support for clinical studies and also consulting fees for serving on advisory boards and as a speaker for Amgen, Ascensia, AstraZeneca, Eli Lilly and Company, Novo Nordisk, Pfizer and Sanofi. He has also received consulting fees from Eli Lilly and Company as a member of the 4B study and of the DURABLE Trial Data Monitoring Committee, and from Novo Nordisk as principal investigator for the SURE Study Netherlands.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. World Health Organization Cost effectiveness and strategic planning (WHO-CHOICE), 2014. Available: https://www.who.int/choice/cost-effectiveness/en/ [Accessed 13 Mar 2019].
- 2. International Diabetes Federation Diabetes atlas. 8th edn, 2017. http://www.diabetesatlas.org/across-the-globe.html [Google Scholar]
- 3. Williams R, Van Gaal L, Lucioni C. Assessing the impact of complications on the costs of type II diabetes. Diabetologia 2002;45:S13–7. 10.1007/s00125-002-0859-9 [DOI] [PubMed] [Google Scholar]
- 4. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). The Lancet 1998;352:837–53. 10.1016/S0140-6736(98)07019-6 [DOI] [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–13. 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gæde P, Lund-Andersen H, Parving H-H, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91. 10.1056/NEJMoa0706245 [DOI] [PubMed] [Google Scholar]
- 7. Gaede P, Valentine WJ, Palmer AJ, et al. Cost-Effectiveness of intensified versus conventional multifactorial intervention in type 2 diabetes: results and projections from the Steno-2 study. Diabetes Care 2008;31:1510–5. 10.2337/dc07-2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aroda VR. A review of GLP-1 receptor agonists: evolution and advancement, through the lens of randomised controlled trials. Diabetes Obes Metab 2018;20((suppl 2)):22–33. 10.1111/dom.13162 [DOI] [PubMed] [Google Scholar]
- 9. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetologia 2018;61:2461–98. 10.1007/s00125-018-4729-5 [DOI] [PubMed] [Google Scholar]
- 10. Netherlands Huisarten Genootschap NHG-Standaard diabetes mellitus type 2 [Accessed 15 Apr 2019].
- 11. Rijksinstituut voor Volksgezondheid en Milieu Diabetes mellitus, 2018. Available: https://www.volksgezondheidenzorg.info/onderwerp/diabetes-mellitus/ [Accessed 13 Mar 2019].
- 12. Peters ML, Huisman EL, Schoonen M, et al. The current total economic burden of diabetes mellitus in the Netherlands. Neth J Med 2017;75:281–97. [PubMed] [Google Scholar]
- 13. American Diabetes Association Consensus Panel Guidelines for computer modeling of diabetes and its complications. Diabetes Care 2004;27:2262–5. 10.2337/diacare.27.9.2262 [DOI] [PubMed] [Google Scholar]
- 14. Palmer AJ, Roze S, Valentine WJ, et al. The core diabetes model: projecting long-term clinical outcomes, costs and Costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(sup1):S5–26. 10.1185/030079904X1980 [DOI] [PubMed] [Google Scholar]
- 15. Palmer AJ, Roze S, Valentine WJ, et al. Validation of the core diabetes model against epidemiological and clinical studies. Curr Med Res Opin 2004;20 Suppl 1:S27–40. 10.1185/030079904X2006 [DOI] [PubMed] [Google Scholar]
- 16. McEwan P, Foos V, Palmer JL, et al. Validation of the IMS core diabetes model. Value in Health 2014;17:714–24. 10.1016/j.jval.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization Global health Observatory data Repository: life tables by country – Netherlands, 2016. Available: http://apps.who.int/gho/data/view.main.60450?lang=en [Accessed 19 Jan 2019].
- 18. Zorginstituut Nederland Guideline for economic evaluations in healthcare, 2016. Available: https://english.zorginstituutnederland.nl/publications/reports/2016/06/16/guideline-for-economic-evaluations-in-healthcare [Accessed 19 Jan 2019].
- 19. Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (sustain 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3A trial. Lancet Diabetes Endocrinol 2017;5:355–66. 10.1016/S2213-8587(17)30085-2 [DOI] [PubMed] [Google Scholar]
- 20. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once Weekly in patients with type 2 diabetes (sustain 7): a randomised, open-label, phase 3B trial. Lancet Diabetes Endocrinol 2018;6:275–86. 10.1016/S2213-8587(18)30024-X [DOI] [PubMed] [Google Scholar]
- 21. Heap G. Type 2 diabetes: current treatment. detailed, expanded analysis (EU 5). available at. Available: www.decisionresourcesgroup.com [Accessed Last accessed November 6, 2018].
- 22. Scottish Medicines Consortium (prepared by Novo Nordisk A/S) Insulin degludec/liraglutide 100 units/mL / 3.6mg/mL solution for injection pre-filled pen (Xultophy®), SMC No. (1088/15), 2015. Available: https://www.scottishmedicines.org.uk/files/advice/insulin_degludec_liraglutide__Xultophy_FINAL_Sept_2015_for_website.pdf [Accessed 13 Dec 2018].
- 23. National Institute for Health and Care Excellence Final appraisal determination: liraglutide for the treatment of type 2 diabetes mellitus, 2011. Available: https://www.nice.org.uk/guidance/ta203/documents/diabetes-liraglutide-final-appraisal-determination3 [Accessed 13 Dec 2018].
- 24. Hunt B, Vega-Hernandez G, Valentine WJ, et al. Evaluation of the long-term cost-effectiveness of liraglutide vs lixisenatide for treatment of type 2 diabetes mellitus in the UK setting. Diabetes Obes Metab 2017;19:842–9. 10.1111/dom.12890 [DOI] [PubMed] [Google Scholar]
- 25. Mezquita-Raya P, Ramírez de Arellano A, Kragh N, et al. Liraglutide versus Lixisenatide: long-term cost-effectiveness of GLP-1 receptor agonist therapy for the treatment of type 2 diabetes in Spain. Diabetes Ther 2017;8:401–15. 10.1007/s13300-017-0239-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunt B, Kragh N, McConnachie CC, et al. Long-Term cost-effectiveness of two GLP-1 receptor agonists for the treatment of type 2 diabetes mellitus in the Italian setting: liraglutide versus Lixisenatide. Clin Ther 2017;39:1347–59. 10.1016/j.clinthera.2017.05.354 [DOI] [PubMed] [Google Scholar]
- 27. Viljoen A, Hoxer CS, Johansen P, et al. Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes Obes Metab 2019;21:611–21. 10.1111/dom.13564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zorginstituut Nederland Medicijnkosten, 2018. Available: https://www.medicijnkosten.nl/ [Accessed 20 Dec 2018].
- 29. Sørensen J, Ploug UJ. The cost of diabetes-related complications: registry-based analysis of days absent from work. Economics Research International 2013;2013:1–8. 10.1155/2013/618039 [DOI] [Google Scholar]
- 30. Beaudet A, Clegg J, Thuresson P-O, et al. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462–70. 10.1016/j.jval.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 31. Evans M, Khunti K, Mamdani M, et al. Health-Related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes 2013;11:90 10.1186/1477-7525-11-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vemer P, Rutten-van Mölken MPMH. Largely ignored: the impact of the threshold value for a QALY on the importance of a transferability factor. Eur J Health Econ 2011;12:397–404. 10.1007/s10198-010-0253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bobinac A, van Exel NJA, Rutten FFH, et al. Willingness to pay for a quality-adjusted life-year: the individual perspective. Value in Health 2010;13:1046–55. 10.1111/j.1524-4733.2010.00781.x [DOI] [PubMed] [Google Scholar]
- 34. Johansen P, Håkan-Bloch J, Liu AR, et al. Cost effectiveness of once-weekly Semaglutide versus once-weekly Dulaglutide in the treatment of type 2 diabetes in Canada. Pharmacoecon Open 2019;127 10.1007/s41669-019-0131-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malkin SJP, Russel-Szymczyk M, Liidemann G, et al. Once-Weekly Semaglutide versus once-daily liraglutide for the treatment of type 2 diabetes: a long-term cost-effectiveness analysis in Estonia. Diabetes Ther 2019;10:159–76. 10.1007/s13300-018-0542-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gæde P JP, Tikkanen CK, Pollock RF, et al. Management of patients with type 2 diabetes with once-weekly Semaglutide versus Dulaglutide, exenatide ER, liraglutide and Lixisenatide: a cost-effectiveness analysis in the Danish setting. Diabetes Ther 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–44. 10.1056/NEJMoa1607141 [DOI] [PubMed] [Google Scholar]
- 38. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. 10.1056/NEJMoa1603827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. The Lancet 2019;394:121–30. 10.1016/S0140-6736(19)31149-3 [DOI] [PubMed] [Google Scholar]
- 40. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. The Lancet 2018;392:1519–29. 10.1016/S0140-6736(18)32261-X [DOI] [PubMed] [Google Scholar]
- 41. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015;373:2247–57. 10.1056/NEJMoa1509225 [DOI] [PubMed] [Google Scholar]
- 42. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–39. 10.1056/NEJMoa1612917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evan M, Johansen P, Vrazic H, et al. The importance of incorporating cardio-protective effects of once-weekly semaglutide. Diabetologia 2018;61(Supplement 1):427–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-000705supp001.pdf (1.5MB, pdf)