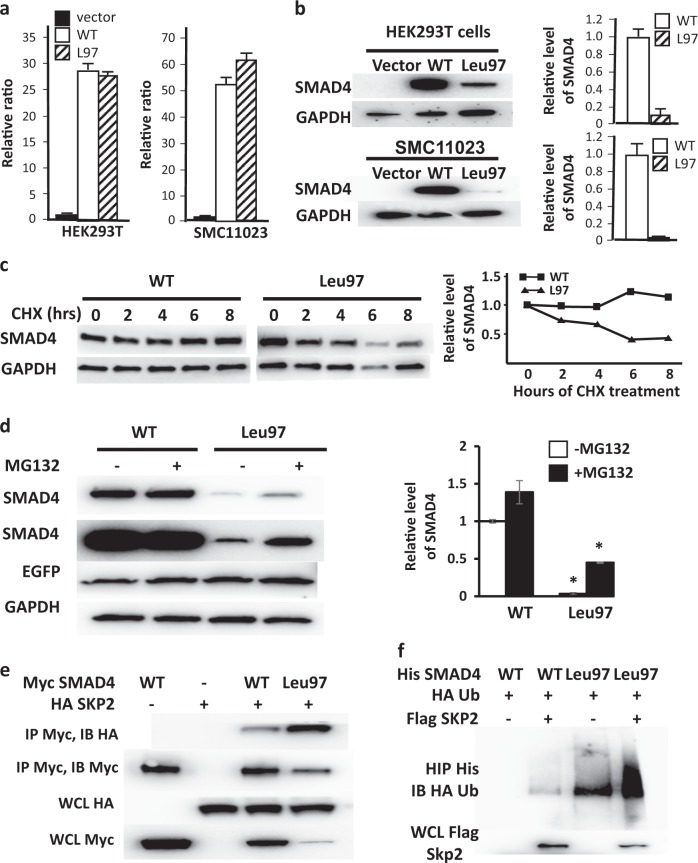
Fig. 2.
SMAD4 Leu97 reduces protein stability through increased binding to SKP2. a Lentiviruses of control vector and Flag-tagged WT and Leu97 SMAD4 were infected in HEK293T and SMC11023 followed by real-time qPCR with GAPDH as internal control. SMAD4 WT and Leu97 have similar expression of mRNA. b In these same cells, the protein level of SMAD4 Leu97 was significantly lower than that of SMAD4 WT. The average protein levels over triplicate experiments are shown on the graph. c SMAD4 Leu97 variant protein is unstable after cycloheximide treatment. HEK293T cells expressing SMAD4 WT and Leu97 variant were treated with cycloheximide for up to 8 h followed by immunoblot analyses with anti-Flag antibodies. The levels of SMAD4 and GAPDH protein were quantified using immunoblot assay. d MG132 partially rescued the degradation of SMAD4 Leu97 variant. HEK293T cells expressing SMAD4 WT and Leu97 variant were treated with MG132, for 8 h. A representative experiment is shown on the left and quantification of three individual experiments are shown at the right. e Leu97 variant increased the ability of SMAD4 binding to HA-SKP2. HEK293T cells were co-transfected with Myc-SMAD4 and HA-SKP2 followed by immunoprecipitation with Myc beads. The SMAD4-bound SKP2 was detected with anti-HA antibodies. f Ubiquitination of SMAD4 Leu97 is increased compared to WT protein. HEK293T cells were co-transfected with His-SMAD4, Flag-SKP2, and HA-ubiquitin followed by immunoprecipitation with Ni-NTA agarose beads under denatured conditions. The ubiquitination of Smad4 was detected with anti-HA antibodies. Three individual experiments were performed and one representative result is shown. Asterisks indicate a p value <0.05