Neurodevelopmental theories of autism, schizophrenia, and other brain disorders strongly implicate the fetal environment1. While certain toxic exposures during pregnancy (infection, malnutrition, urbanicity) have been repeatedly associated with disease risk, specific etiologic mechanisms remain unclear. It is likely, though, that alterations in the fetal methylation milieu are centrally important.
Methylation reactions contribute directly to DNA synthesis, and are thus essential to central nervous system development. Further, methylation of histones and DNA regulates gene expression in both fetal and postnatal life2. While DNA methylation fluctuates greatly during fetal development, methylation marks from late in gestation tend to be enduring3. As such they could influence expression of risk-relevant genes during or long after fetal development1. However, identification of specific fetal epigenetic patterns that underlie neuropsychiatric risk is challenging for numerous reasons – chiefly, the inability to measure and manipulate such patterns directly in living human fetal brains, and the difficulty of relating fetal exposure to risk of illness years later.
That said, quasi-experimental, population-based studies of prenatal folic acid exposure offer largely untapped potential to probe how variation in the fetal methylome influences disease risk. Folate, an essential B-vitamin found in leafy green vegetables, citrus, and lentils supplies one-carbon (methyl) moieties that drive methylation reactions in the body. A synthetic and more highly bioavailable form of folate, folic acid, is found in vitamin supplements as well as in some enriched grain products. Studies in the 1980s conclusively linked low maternal folate levels early in pregnancy with risk for spina bifida and other neural tube defects (NTDs). Based on this evidence, and on the fact that such defects occur before many pregnancies are recognized, it is broadly recommended that all women of childbearing age consume daily folic acid through vitamin supplements. Further, 81 countries now mandate folic acid fortification of enriched grain products. Within the US, this intervention (introduced in 1996) not only reduced NTD incidence, but more broadly, it rapidly doubled blood folate levels in women of child-bearing age4.
However, there remains marked variation in maternal folic acid intake, relating to both fortification exposure and timing of supplement use. Reasons for this variation are likely complex, reflecting patient and clinician knowledge and a range of individual, practical, and geographic factors. In spite of this heterogeneity, carefully conducted studies that relate prenatal folic acid intake to psychiatric outcomes have produced impressive, if correlational findings. Several large, prospective cohort studies have associated periconceptional use of folic acid supplements – i.e., initiation of supplements either before conception or within the first 8 weeks thereafter – with sizable reductions (nearly 50%) in the offspring’s subsequent risk for autism (see, e.g.,5, but note one study with null findings6). Conversely, replicated studies demonstrate a transient doubling of schizophrenia incidence two decades after prenatal exposure to famine. While it is impossible to isolate specific causal factors from among many associated with starvation, the co-occurrence of NTDs in one such cohort suggests low prenatal folate as a parsimonious explanation7. Further, studies of folic acid supplementation in adult schizophrenia patients (e.g.,8) demonstrate modest clinical benefit, although with effect sizes considerably smaller than in prenatal studies.
This work raises important questions about how – and when – folic acid may protect against neurodevelopmental disorders. For NTDs, a plausible explanation relates to the dependence of neuroepithelium that overlies the neural tube, which closes around pregnancy day 28, on one-carbon moieties for DNA synthesis. However, it is curious and somewhat counterintuitive that periconceptional (rather than later) folic acid supplement use should influence risk for autism and potentially schizophrenia. These disorders are characterized by subtle, primarily microscopic abnormalities in brain structure and function. But development of brain tissue in the first two months of pregnancy is comparably primitive, largely comprising proliferation and migration of neural progenitor cells; disruption of this process results in gross abnormalities that are frequently incompatible with life. Resolving this apparent contradiction could provide opportunities not only to substantiate epidemiologic correlations with underlying mechanisms, but also to discover new avenues for early intervention.
One potential explanation for this temporal discrepancy, proposed herein, relates to the effect of pregnancy itself on maternal folate levels (Figure). Before the supplementation era, it was observed that fetal demand for DNA synthesis and chromatin methylation throughout pregnancy dramatically reduces maternal folate levels, which are lowest at childbirth9. Further, studies of sequential folate depletion and repletion in young women indicate that recovery of serum folate levels takes weeks to months to achieve10. Even with periconceptional exposure to fortification, and/or folic acid supplements initiated later in the first trimester, the delayed recovery of maternal folate levels may be insufficient to protect against risk for the more subtle developmental abnormalities of schizophrenia and autism (e.g., in arborization and cortical specialization), which likely occur late in pregnancy. In contrast, periconceptional supplements may provide sufficient reserves to protect against both NTDs and neuropsychiatric risk.
Figure. Maternal folate levels and risk for pre- versus postnatal-onset CNS disorders.
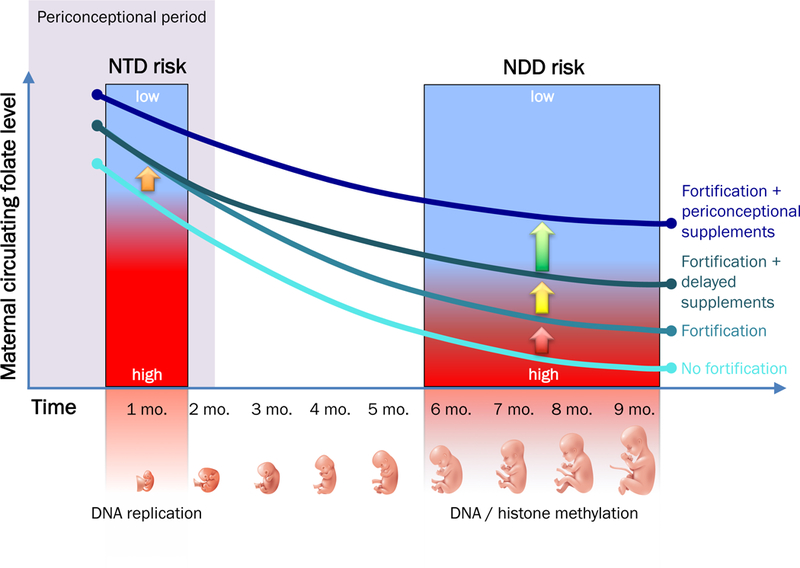
Rapidly dividing fetal and placental cells, and their related demand for one-carbon moieties to support DNA synthesis and DNA/histone methylation, cause maternal blood folate levels to decline throughout pregnancy. In the setting of low periconceptional maternal folate levels, this decline can have adverse consequences on CNS development. Epidemiologic evidence suggests that risk for neural tube defects (NTD) and, potentially, for more subtle neurodevelopmental disorders (NDD) such as autism and schizophrenia can be mitigated by maternal intake of food-based folic acid (fortification) or prenatal vitamins containing folic acid (supplements). However, the type and timing of this exposure may have different implications for NTD versus NDD risk. The curves in this figure represent four different levels of exposure, their effects on circulating folate levels throughout pregnancy, and their potential relevance to prevention. Exposure to fortification alone (orange arrow) may be sufficient to ameliorate NTD risk, even in the absence of prenatal vitamin supplements during neural tube closure in the first month of pregnancy. However, even on the background of fortification exposure (red arrow), delay of supplements until after the periconceptional period (yellow arrow) may not sufficiently restore depleted maternal folate stores in time for optimal epigenetic priming later in gestation, when specialized brain systems develop. As DNA and histone methylation marks established in fetal life persist well after birth, periconceptional folate deficits potentially predispose to altered cortical development throughout childhood and adolescence, and increased risk for NDD.
While this and other mechanistic hypotheses remain unproven, this knowledge gap need not delay additional clinical and epidemiologic studies of prenatal folic acid and neuropsychiatric risk. For example, it will be of interest to monitor incidence of severe mental illness in coming years, as the first cohort of youth exposed to mandatory folic acid fortification in utero now approach the age of greatest risk for these illnesses.
More broadly, leveraging fetal folic acid exposure to study fetal methylomics provides an unusual opportunity to conduct low-risk, potentially high-reward studies in human developmental neuroscience. There is overwhelming evidence that recommended doses of periconceptional folic acid supplements confer minimal risk to the mother or fetus. As such, further studies of any potential neuroprotective benefit, even if small, are easily justified – especially in light of the low cost and ready availability of folic acid supplements. At the same time, non-invasive, translational studies of prenatal folic acid exposure and related epigenetic changes (e.g., using human stem cell models) may reveal molecular, cellular, and systems-level mechanisms through which fetal methylation influences subsequent risk of brain disease. Such work promises both fundamental insights into prenatal brain development and the possibility of preventing at least some cases of severe mental illness in young people.
Acknowledgments
Supported by NIMH, R01MH101425 (J.L.R.) NIMH had no role in preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication. The author reports receipt of consulting honoraria from Pamlab for unrelated projects.
References
- 1.Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68(4):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irwin RE, Pentieva K, Cassidy T, et al. The interplay between DNA methylation, folate and neurocognitive development. Epigenomics. 2016;8(6):863–879. [DOI] [PubMed] [Google Scholar]
- 3.Numata S, Ye T, Hyde TM, et al. DNA methylation signatures in development and aging of the human prefrontal cortex. Am J Hum Genet. 2012;90(2):260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeiffer CM, Hughes JP, Lacher DA, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr. 2012;142(5):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013;309(6):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virk J, Liew Z, Olsen J, Nohr EA, Catov JM, Ritz B. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016;20(6):710–718. [DOI] [PubMed] [Google Scholar]
- 7.Susser E, St Clair D. Prenatal famine and adult mental illness: interpreting concordant and discordant results from the Dutch and Chinese Famines. Soc Sci Med. 2013;97:325–330. [DOI] [PubMed] [Google Scholar]
- 8.Roffman JL, Lamberti JS, Achtyes E, et al. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. 2013;70(5):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball EW, Giles C. Folic Acid and Vitamin B12 Levels in Pregnancy and Their Relation to Megaloblastic Anaemia. J Clin Pathol. 1964;17:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shelnutt KP, Kauwell GP, Gregory JF 3rd, et al. Methylenetetrahydrofolate reductase 677C-->T polymorphism affects DNA methylation in response to controlled folate intake in young women. J Nutr Biochem. 2004;15(9):554–560. [DOI] [PubMed] [Google Scholar]


