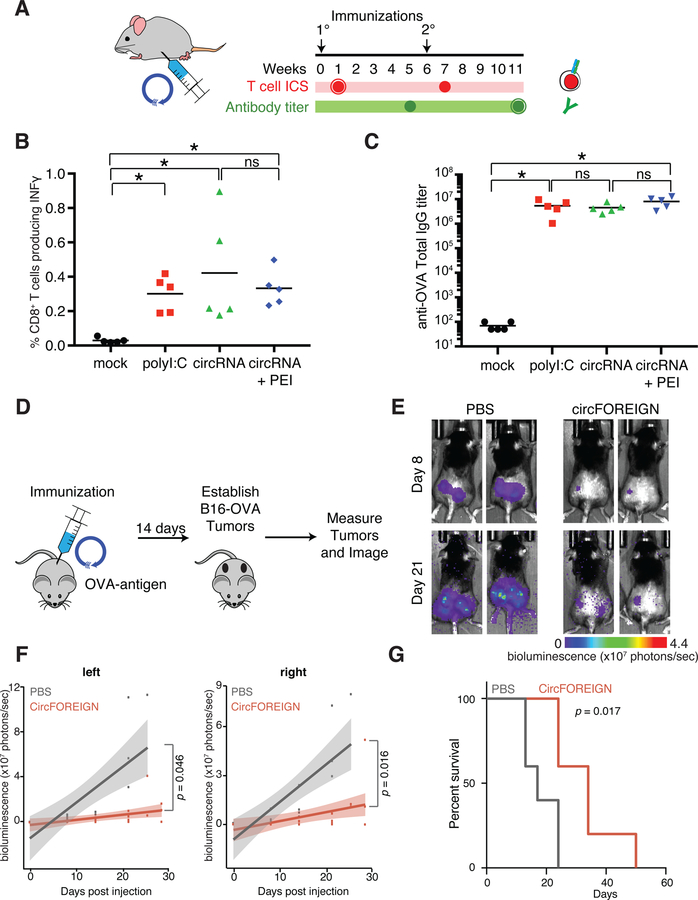
Figure 1. CircFOREIGN induces immune response but m6A-modified circFOREIGN attenuates stimulation in vivo.
A. Agonist RNA in conjunction with OVA is delivered by subcutaneous injection. T cell ICS and antibody titers are measured at the indicated times following primary and secondary immunizations.
B. CircRNA stimulates anti-OVA T cell responses independent of transfection agent following primary vaccination. Means are shown (n = 5), *p < 0.05, Kruskal-Wallis test.
C. CircRNA stimulates anti-OVA antibody titers independent of transfection agent following secondary vaccination. Means are shown (n = 5), *p < 0.05, Anova-Tukey’s test.
D. CircFOREIGN vaccination in conjunction with OVA is delivered by subcutaneous injection. 14 days later, OVA-expressing B16-melanoma cells are established in right and left flanks. Tumors are measured and imaged.
E. Quantification of bioluminescence measurements in left and right tumors for mice vaccinated with PBS or circFOREIGN prior to tumor establishment. p value calculated by Wilcoxon signed-rank test. n=5 mice in each group.
F. Quantification of bioluminescence measurements in left and right tumors for mice vaccinated with PBS or circFOREIGN prior to tumor establishment. p value calculated by Wilcoxon signed-rank test. n=5 mice in each group.
G. Mice vaccinated with circFOREIGN survive twice as long as negative control mice. Survival curves for mice vaccinated with PBS or circFOREIGN prior to tumor establishment. p value calculated by log-rank test. n=5 mice in each group.