Figure 4.
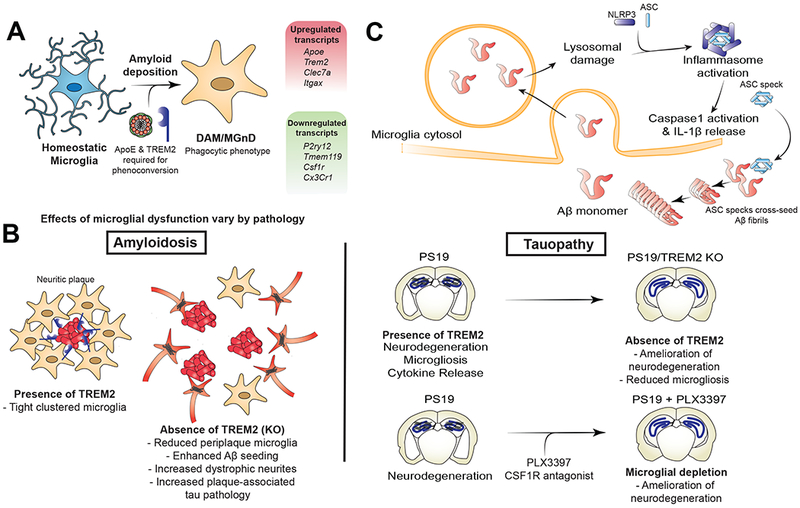
Selected roles of the innate immune system in AD pathophysiology. (A) Transcriptomic analyses of microglia isolated from mice with amyloid pathology have demonstrated a unique microglial subpopulation (DAM/MGnD) only found in diseased animals and defined by reduced expression of homeostatic genes and increased expression of genes involved in phagocytosis and microglial activation. Expression of ApoE and TREM2 is necessary for the development of this disease-specific subpopulation. (B) Microglial activation and phagocytosis have disparate effects based on the prominent pathology studied. TREM2 KO results in a microglial phenotype that is less activated and less phagocytic. In mouse models of amyloid deposition, TREM2 KO leads to reduced peri-plaque microglial clustering, increased Aβ seeding and deposition, and increased dystrophic neurites with enhanced plaque-associated tau pathology. Conversely, in PS19 tauopathy mice, TREM2 KO leads to reduced neurodegeneration and microgliosis. Similarly, near total microglial depletion in PS19 mice expressing ApoE4 leads to significant reduction in neurodegeneration and microgliosis. (C) Aβ phagocytosis leads to increased microglial activation. Lysosomal damage by Aβ can lead to activation of NLRP3 inflammasome, resulting in IL-1β secretion. Extracellular ASC specks released from activated microglial can then further cross-seed fibrillization of Aβ into fibrils.
